Dr. @marinagarassino presents 5-year efficacy and safety update of KEYNOTE-189 (1L carboplatin plus pemetrexed +- pembrolizumab in non-squamous NSCLC) #ESMO22 

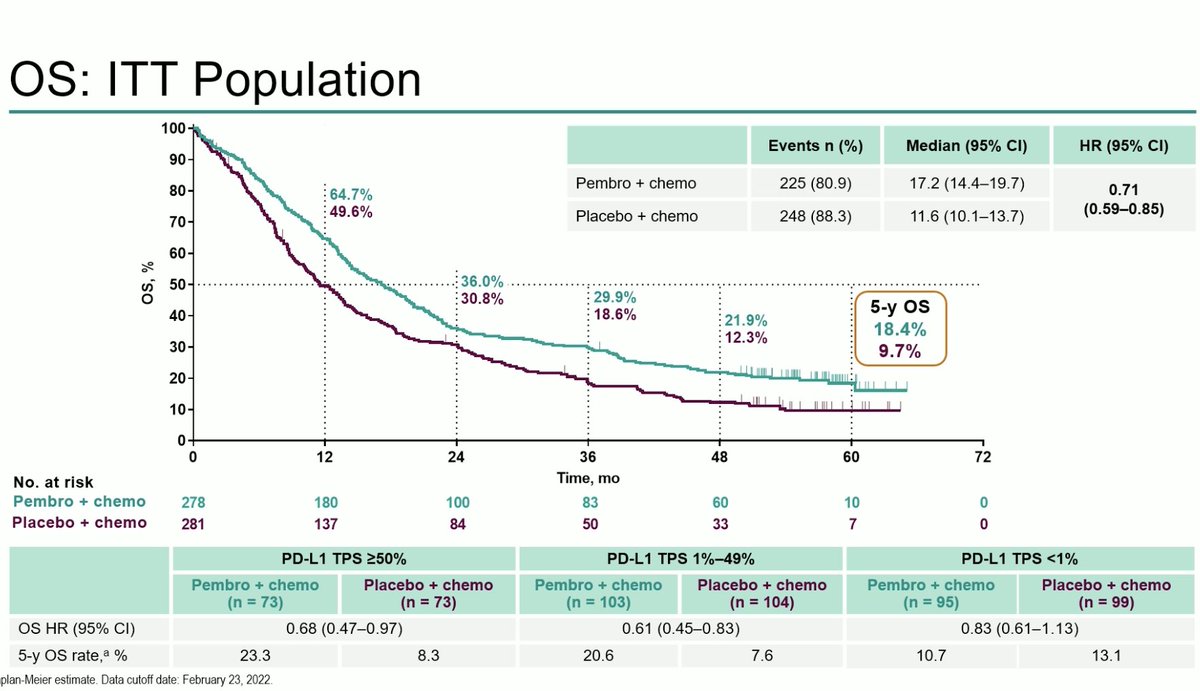
KEYNOTE 189 is our SOC and has shown a consistent benefit including improving OS even with a crossover rate of 57%. #ESMO22 
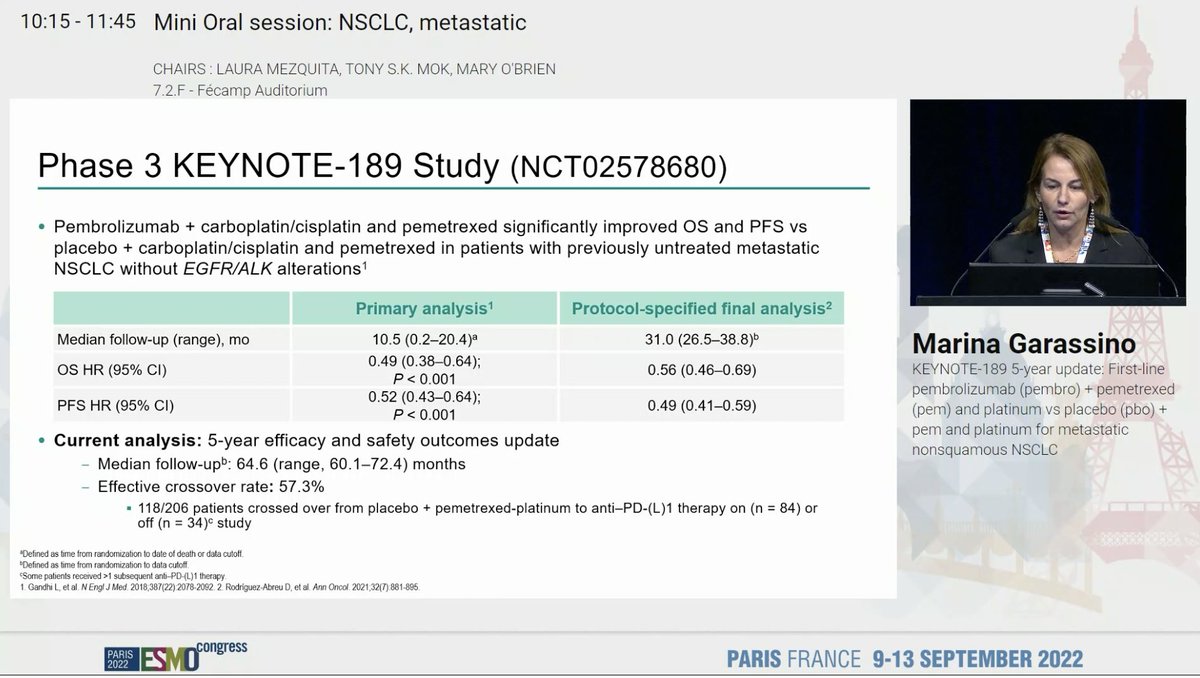
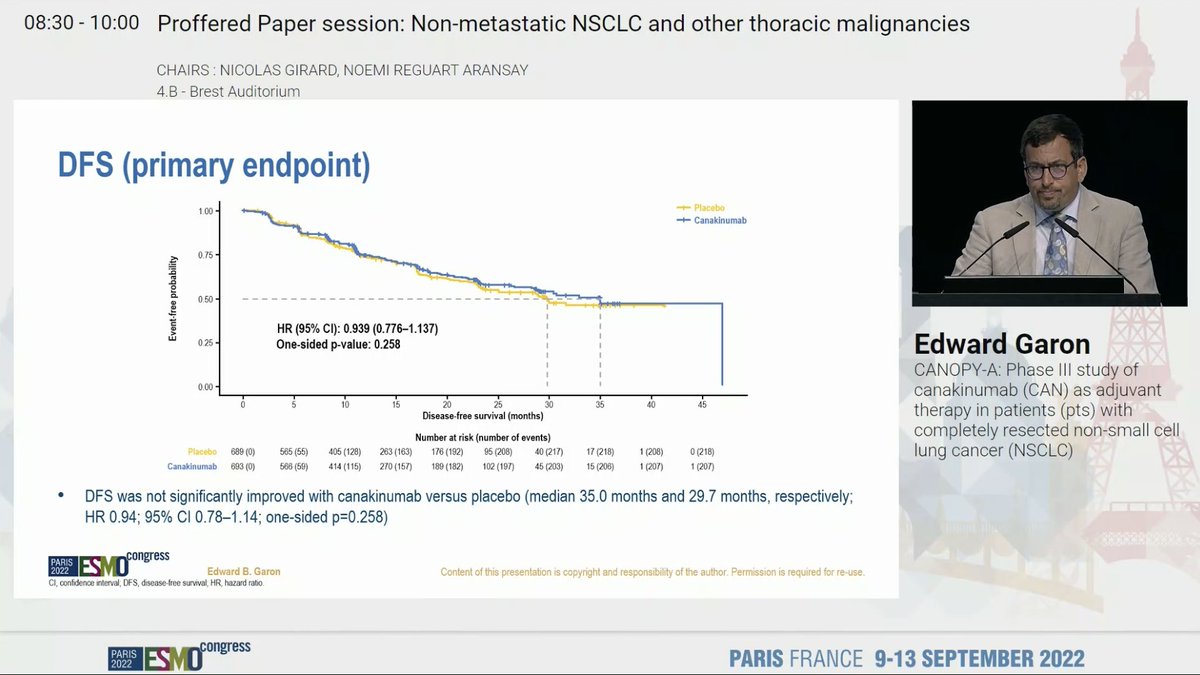
KEYNOTE 189 shows sustained OS benefit with longer follow up and a 5y OS rate of 18.4% with an OS HR of 0.60. Better PFS and OS also observed. Benefit across PDL1 strata. No new safety signals . #ESMO22 







Here are data from patients who completed the 35 planned cycles of pembrolizumab on KEYNOTE 189. In that group, RR 86% and mDOR 58m with a 72% 3y OS rate. Note 40% alive without PD or subsequent therapy. Looking forward to more details on this group. #ESMO22 

Longer follow up confirms survival benefit of chemo-immunotherapy in NSCLC. Important update from #ESMO22 by @marinagarassino 

• • •
Missing some Tweet in this thread? You can try to
force a refresh