🧵Your 18-year-old patient has severe hemophilia B, no history of inhibitors, and has low titer AAV antibodies. He has been treated on-demand for the past 5 years. Would he be a candidate for gene therapy currently in clinical trials? #Tweetorial #MedEd #GeneTherapy #Hemophilia
Understanding who might be a candidate for gene therapy for #hemophilia (GTH) is critical. Why? This new therapy has been approved by regulatory authorities in Europe and is under consideration in the US. So who is eligible? Review: genetherapy.isth.org/9153-tweetoria… #cme_accreditation 

First, How would you characterize your interest in hemophilia?
Before jumping in, how does GTH work? For recently approved therapy, viral vectors carry an edited copy of DNA for the defective protein in hemophilia A (FVIII) or B (FIX) to the liver where they enter hepatocytes and initiate synthesis of working protein. pubmed.ncbi.nlm.nih.gov/30710128/ 

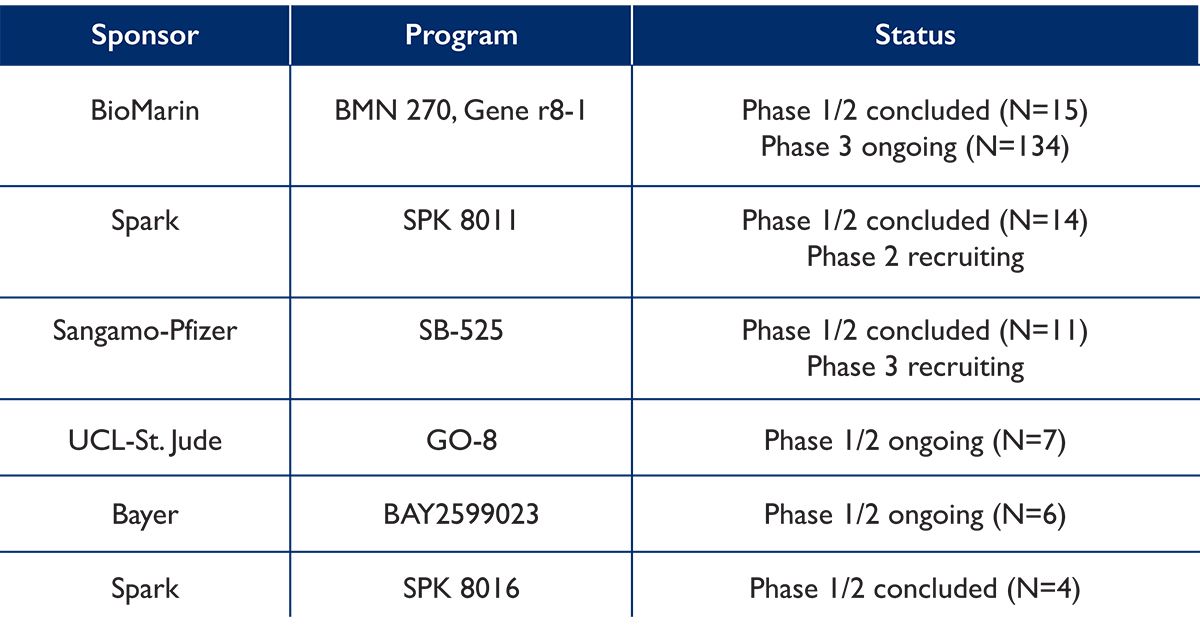
Not all patients with hemophilia will be candidates. Why? This method of GTH has limitations which are reflected in inclusion and exclusion criteria for the clinical trials, so not all patients with hemophilia will be eligible. Let’s take a closer look. pubmed.ncbi.nlm.nih.gov/32490590/ 

Initially, children and patients with mild to moderate disease will not be candidates. Why? Current clinical trials are testing GTH in adult patients (≥18 years) with severe hemophilia, defined as ≤1 IU/dL for hemophilia A and <2 IU/dL for hemophilia B. 

Patients treated on-demand may not be candidates. Why? Most patients in clinical trials have been on prophylactic treatment with either FVIII or FIX for a minimum of 150 days.
A specific blood test is needed to detect preexisting antibodies to the viral vector being used to transfer the therapeutic DNA into liver cells. In most of the current GTH clinical trials, patients with these antibodies have been excluded from participation. 

Patients with a history of FVIII or FIX inhibitors or those with detectable levels at screening for participation is an additional exclusion criteria for GTH clinical trials.
Liver dysfunction (indicated by cirrhosis, fibrosis, and/or AST or ALT level >2 x ULN), the presence of hepatitis B or C, and HIV infection have also been included in the exclusion criteria for GTH clinical trials.
Conversations among patients, providers, and caretakers will be essential to treatment decisions. In addition to inclusion and exclusion criteria, factors related to safety, current treatment options, and quality of life must be considered. pubmed.ncbi.nlm.nih.gov/34232980/
Let’s see what you learned. Consider your 18-year-old patient with severe hemophilia B. He has no history of inhibitors, low titer AAV antibodies, and has been treated on-demand for the past 5 years. Would he be a candidate for gene therapy currently in clinical trials?
The correct answer is D. For more detailed inclusion and exclusion criteria as well as additional treatment considerations for patients with hemophilia and caregivers contemplating GTH, go to
genetherapy.isth.org/education
genetherapy.isth.org/education
• • •
Missing some Tweet in this thread? You can try to
force a refresh