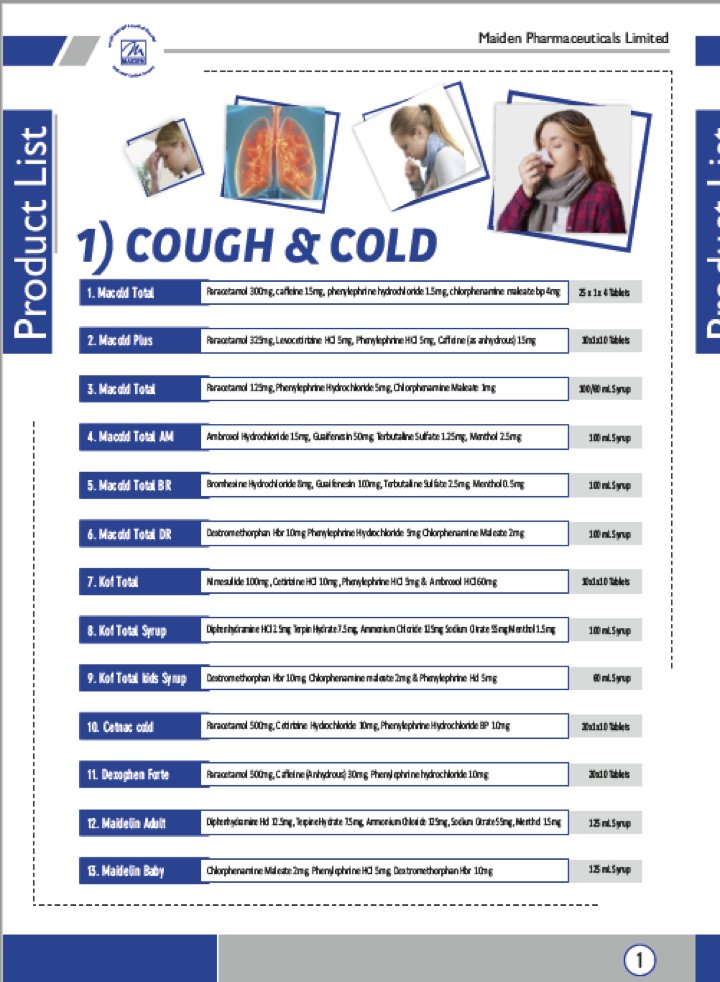
Our book, 'The Truth Pill: The Myth of Drug Regulation in India' is available for purchase on Amazon starting today:
amazon.in/TRUTH-PILL-Myt…
Its a product of many years of research and over 400 RTI responses.
1/n
#truthpill @Preddy85 @SimonSchusterIN
amazon.in/TRUTH-PILL-Myt…
Its a product of many years of research and over 400 RTI responses.
1/n
#truthpill @Preddy85 @SimonSchusterIN

2/2
We wanted to provide our readers direct access to our research because there is only so much we could put into our book. To that end, we are announcing the launch of an accompanying website for #thetruthpill where all our research is curated.
thetruthpill.in
We wanted to provide our readers direct access to our research because there is only so much we could put into our book. To that end, we are announcing the launch of an accompanying website for #thetruthpill where all our research is curated.
thetruthpill.in

3/n
All primary research for our book is available to journalists, reporters and anyone who is interested in studying this topic further can access it here:
thetruthpill.in/resources/
All primary research for our book is available to journalists, reporters and anyone who is interested in studying this topic further can access it here:
thetruthpill.in/resources/

5/n
Our hope is that newsrooms, independent journalists, researchers and anyone who is interested in the topic of Drug Regulation in India can use this archive for their own research.
@nit_set @samar11 @tajmahalfoxtrot @ShekharGupta @svaradarajan @VikasReports @Moneylifers
Our hope is that newsrooms, independent journalists, researchers and anyone who is interested in the topic of Drug Regulation in India can use this archive for their own research.
@nit_set @samar11 @tajmahalfoxtrot @ShekharGupta @svaradarajan @VikasReports @Moneylifers
6/n
Over the years, we have sent many petitions to the @MoHFW_INDIA asking for transparency, accountability, comments on proposed amendments etc. These are part of our advocacy for a better regulated medicines supply chain in India.
casemindia.org/petitions/
Over the years, we have sent many petitions to the @MoHFW_INDIA asking for transparency, accountability, comments on proposed amendments etc. These are part of our advocacy for a better regulated medicines supply chain in India.
casemindia.org/petitions/
7/n
You will find Parliamentary Reports, Government Reports on Drug Regulation here 👇which is a good companion to the research material for our book
casemindia.org/government-rep…
You will find Parliamentary Reports, Government Reports on Drug Regulation here 👇which is a good companion to the research material for our book
casemindia.org/government-rep…
• • •
Missing some Tweet in this thread? You can try to
force a refresh