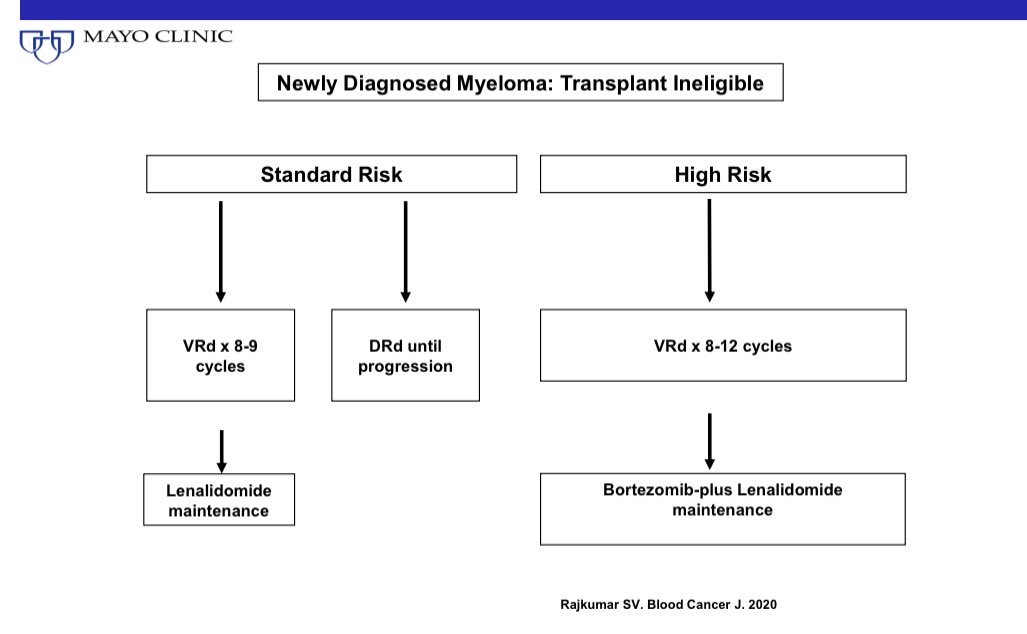
What is the best treatment for newly diagnosed myeloma in transplant ineligible patients?
VRd or DRd?
There’s a lot to learn beyond myeloma by studying this question. #medtwitter @ESHaematology
1/
VRd or DRd?
There’s a lot to learn beyond myeloma by studying this question. #medtwitter @ESHaematology
1/

Both triplets VRd and DRd are excellent and well tolerated. Both have beaten lenalidomide/dexamethasone (Rd) in randomized trials.
One adds bortezomib to Rd —-> (VRd regimen)
2/
One adds bortezomib to Rd —-> (VRd regimen)
2/

Unfortunately no randomized trial has compared VRd and DRd head to head.
So we are forced to compare data from two phase III trials. On the surface DRd seems to do better — but there’s more than meets the eye.
4/
So we are forced to compare data from two phase III trials. On the surface DRd seems to do better — but there’s more than meets the eye.
4/
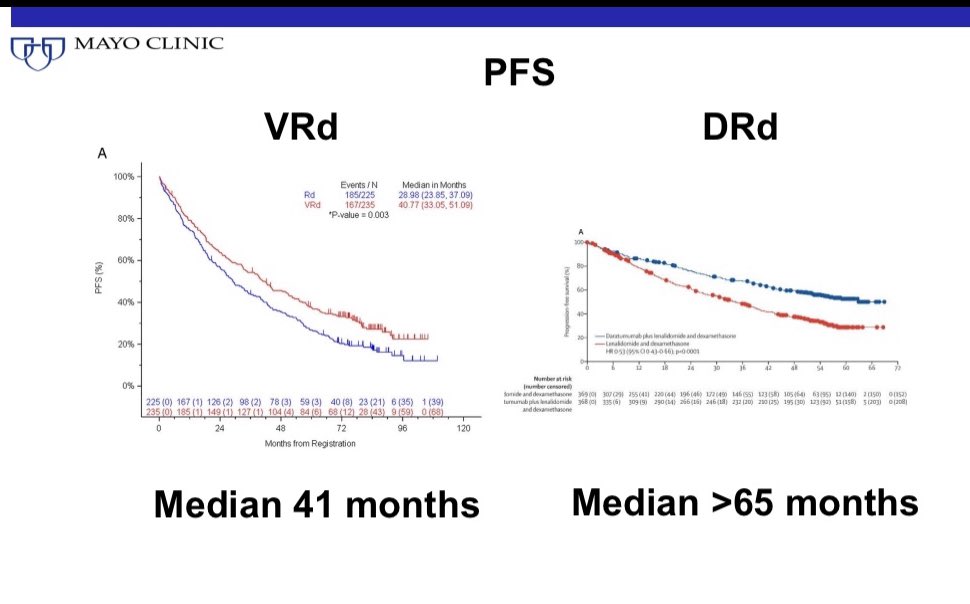
These trials were done in different eras with different salvage options. One trial was done in the US. The other mainly outside the US.
DRd was studied in a population age 65+. Only 40% in the VRd trial were 65+.
So how do we compare?
5/
DRd was studied in a population age 65+. Only 40% in the VRd trial were 65+.
So how do we compare?
5/
First is to not to be swayed too much by PFS differences.
With DRd the PFS will naturally be longer because the regimen uses all 3 drugs for years and years until progression.
VRd uses 3 drugs for 6 months and then only oral therapy with 2 drugs (Rd).
6/
With DRd the PFS will naturally be longer because the regimen uses all 3 drugs for years and years until progression.
VRd uses 3 drugs for 6 months and then only oral therapy with 2 drugs (Rd).
6/
Overall survival of patients aged 65+ is similar between the two regimens.
The 5 year survival rate is better for DRd but there were a lot more salvage options available in the period DRd was studied than when VRd was studied.
7/
The 5 year survival rate is better for DRd but there were a lot more salvage options available in the period DRd was studied than when VRd was studied.
7/
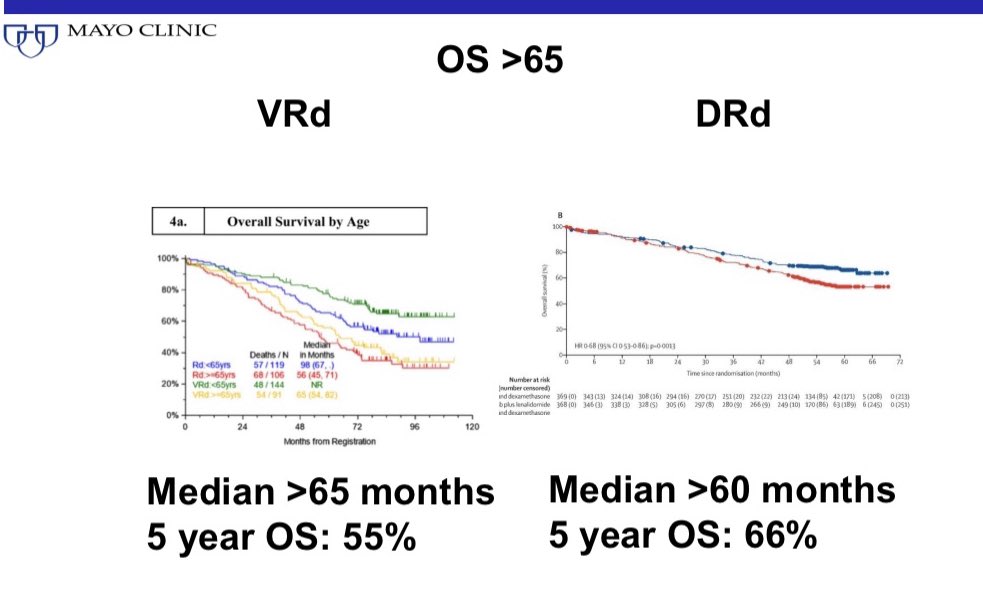
So we are left with two excellent regimens with excellent outcomes and no major differences in efficacy beyond what’s expected given the differences in time periods of the trials and the schedule of treatment used.
So how do we decide?
8/
So how do we decide?
8/
This situation where we have to decide best treatment with inadequate and imperfect data confronts us frequently in medicine.
Besides efficacy I also look at toxicity, convenience, cost, access, specific patient factors to decide.
9/
Besides efficacy I also look at toxicity, convenience, cost, access, specific patient factors to decide.
9/
The unique toxicity of VRd is risk of neuropathy. But with 6 months weekly SQ bortezomib the risk of irreversible neuropathy is low.
DRd is well tolerated. The main concern will be prolong daratumumab can suppress the immune system and increase risk of infections.
10/
DRd is well tolerated. The main concern will be prolong daratumumab can suppress the immune system and increase risk of infections.
10/
In terms of convenience, VRd wins handily because it is only oral therapy after the first 6 months. DRd requires monthly SQ or IV Daratumumab until progression.
The side effects of prolonged doublet will always be lower than when you add an extra drug to the same doublet.
11/
The side effects of prolonged doublet will always be lower than when you add an extra drug to the same doublet.
11/
In terms of cost, it’s no contest. VRd can be administered for $100-200 a month in most parts of the world. Total $1000-10,000 per year.
DRd will cost 10-100 times more: $100,000 to $200,000 a month.
Note the cost in the US for both regimens is much higher.
12/
DRd will cost 10-100 times more: $100,000 to $200,000 a month.
Note the cost in the US for both regimens is much higher.
12/
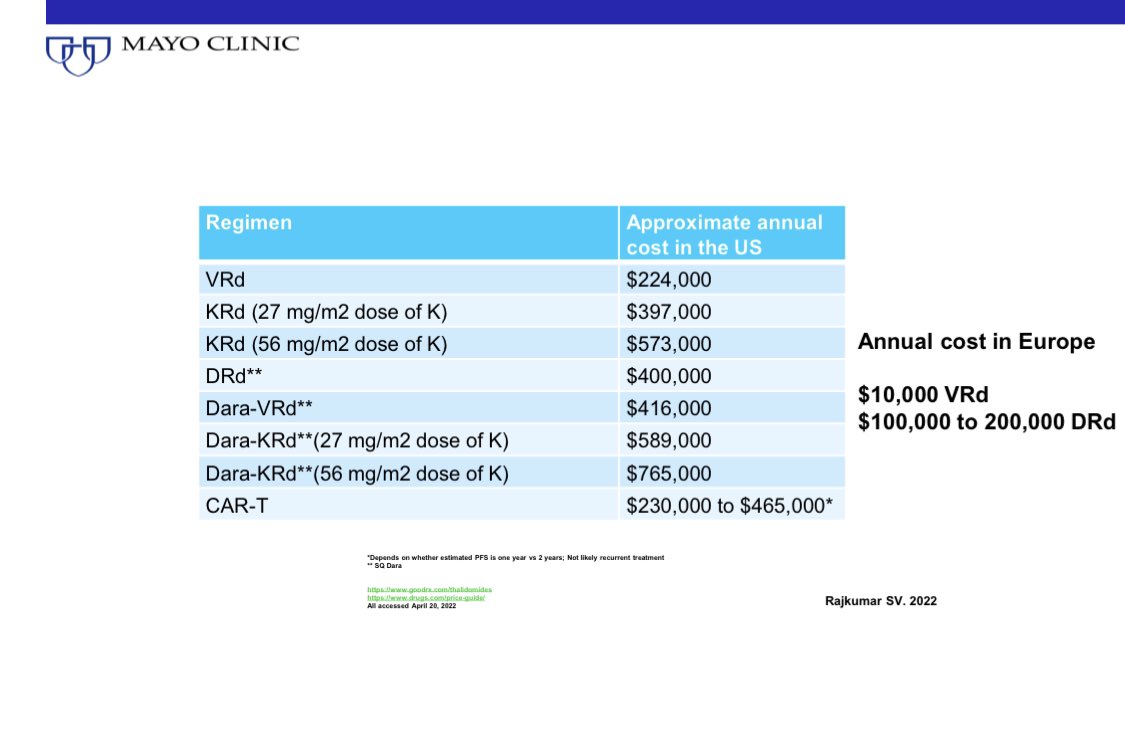
Patient factors are also a consideration. Eg., If patients have underlying neuropathy, DRd is preferred.
13/
13/
In high risk patients, I prefer continuing bortezomib every other week indefinitely as maintenance in addition to Lenalidomide. So the advantage of only using oral therapy long term is no longer there. Either VRd or DRd are ok.
14/
14/
In summary both VRd and DRd are excellent regimens. One is not second rate compared to the other.
I prefer VRd for most patients because of the lower cost, and greater convenience. But DRd if available and affordable is fine. But you must discuss the trade offs with the patient
I prefer VRd for most patients because of the lower cost, and greater convenience. But DRd if available and affordable is fine. But you must discuss the trade offs with the patient
More discussion and references here. @BloodCancerJnl nature.com/articles/s4140…
• • •
Missing some Tweet in this thread? You can try to
force a refresh