Quick follow up thread on the #coughsyrup tragedy in The Gambia.
The official line seems to be that we dont have to worry about adulterated cough syrup because the company is making this product just for its overseas customers.
Lets see if this holds water, shall we?
1/n
The official line seems to be that we dont have to worry about adulterated cough syrup because the company is making this product just for its overseas customers.
Lets see if this holds water, shall we?
1/n
3/n
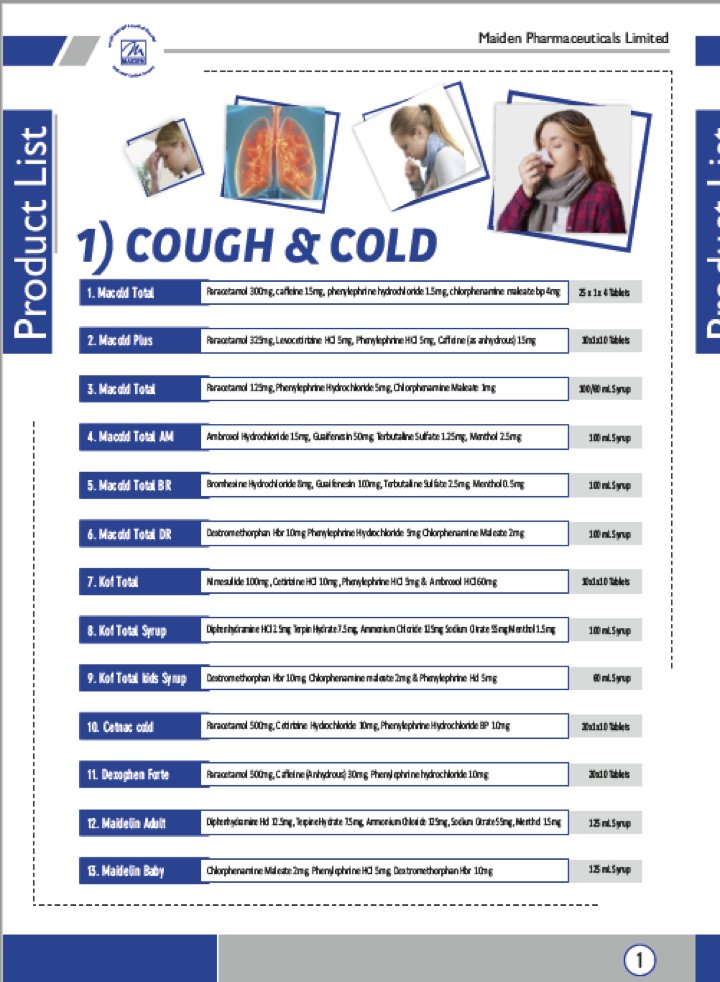
While they may not selling cough syrup under the names they exported it to the Gambia, they are likely selling cough-syrup in India.
Here are some of their products – we found domestic trademark registrations for Kof-Total which likely means they are selling it in India:
While they may not selling cough syrup under the names they exported it to the Gambia, they are likely selling cough-syrup in India.
Here are some of their products – we found domestic trademark registrations for Kof-Total which likely means they are selling it in India:
8/n
What do you think about the claim made by him 👇👇 now?
What do you think about the claim made by him 👇👇 now?
https://twitter.com/ANI/status/1578641909723332609?s=20&t=qiZJYIdONKNf1l-xvwxxjA
9/n
More importantly, why do you think the administration put out a press release giving credence to this argument?
Shouldnt you ask?
The reason we should is because it has happened before.
Stay with me please.
More importantly, why do you think the administration put out a press release giving credence to this argument?
Shouldnt you ask?
The reason we should is because it has happened before.
Stay with me please.
10/n
Some eight months after the first wave of deaths due to DEG poisoning in Jammu in 2020, a two year old child, Radhika succumbed to the same cause after taking a syrup manufactured by Digital Vision sold under a different name.
Some eight months after the first wave of deaths due to DEG poisoning in Jammu in 2020, a two year old child, Radhika succumbed to the same cause after taking a syrup manufactured by Digital Vision sold under a different name.
11/n
@PriyankaPulla did a heart-wrenching report on this incident here:
livemint.com/politics/polic…
Why is the CDSCO not initiating a nation-wide recall for all syrups made by Maiden Pharma is a question that we need to ask?
@PriyankaPulla did a heart-wrenching report on this incident here:
livemint.com/politics/polic…
Why is the CDSCO not initiating a nation-wide recall for all syrups made by Maiden Pharma is a question that we need to ask?
12/n
Read about our dysfunctional drug regulatory system and how broken the whole process is which leads to deaths of young children like Radhika every day in the #truthpill
thetruthpill.in
Read about our dysfunctional drug regulatory system and how broken the whole process is which leads to deaths of young children like Radhika every day in the #truthpill
thetruthpill.in

• • •
Missing some Tweet in this thread? You can try to
force a refresh