Thread 👇
Thread
As @Preddy85 and I were about to begin the session for the launch of the #truthpill this past Saturday evening, like clockwork, we received an email from the @CDSCO_INDIA_INF with a menacing notice threatening
1/n
Thread
As @Preddy85 and I were about to begin the session for the launch of the #truthpill this past Saturday evening, like clockwork, we received an email from the @CDSCO_INDIA_INF with a menacing notice threatening
1/n
2/n
“to exhaust every available recourse including all possible legal options to take action to dissuade us” from repeating certain comments we made in our interview published in the India Today Interview:
indiatoday.in/india/story/ki…
“to exhaust every available recourse including all possible legal options to take action to dissuade us” from repeating certain comments we made in our interview published in the India Today Interview:
indiatoday.in/india/story/ki…
7/n
Clearly there must be some very hardworking bureaucrats in the CDSCO to have timed their notice for 6 pm on a Saturday evening!
We did respond to this notice earlier today. The primary complaint of the CDSCO appears to be the statements we made stating that
Clearly there must be some very hardworking bureaucrats in the CDSCO to have timed their notice for 6 pm on a Saturday evening!
We did respond to this notice earlier today. The primary complaint of the CDSCO appears to be the statements we made stating that
8/n
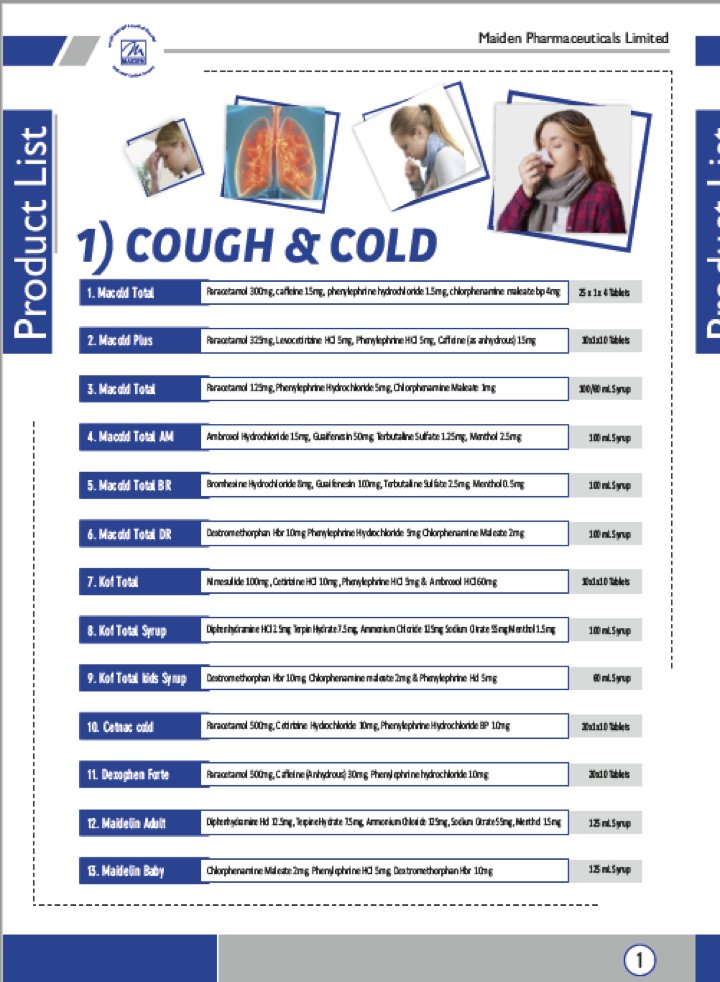
they could not escape responsibility for the tragedy in Gambia by blaming it entirely on the state drug regulator because the manufacturing facility in question comes up on the list of COPP-GMP facilities published by the CDSCO.
they could not escape responsibility for the tragedy in Gambia by blaming it entirely on the state drug regulator because the manufacturing facility in question comes up on the list of COPP-GMP facilities published by the CDSCO.
9/n
There is a whole lot of confusion amongst reporters about how the Certificate of Pharmaceutical Product (COPP) is issued by the Govt., so here is a short thread.
To begin with a COPP is a certification scheme created by the @WHO for certifying and building trust in exports.
There is a whole lot of confusion amongst reporters about how the Certificate of Pharmaceutical Product (COPP) is issued by the Govt., so here is a short thread.
To begin with a COPP is a certification scheme created by the @WHO for certifying and building trust in exports.
10/n
The system is almost self-regulated by countries with WHO only playing a role in laying down the framework and documentation required. Compliance is largely left to the buyer (buying nation) other than the assurance of the world health body.
The system is almost self-regulated by countries with WHO only playing a role in laying down the framework and documentation required. Compliance is largely left to the buyer (buying nation) other than the assurance of the world health body.
11/n
The WHO’s qualification criteria to participate in the COPP programs is very specific. :
Here is the link: who.int/teams/regulati…
Look closely at bullet point #3 below:
The WHO’s qualification criteria to participate in the COPP programs is very specific. :
Here is the link: who.int/teams/regulati…
Look closely at bullet point #3 below:

12/n
It clearly requires the program to be administered by national drug regulators -India has only one national regulator – the CDSCO
Now, in June 2009, at the 40th meeting of the Drugs Consultative Committee (DCC), it was decided that the Drug Controller General of India
It clearly requires the program to be administered by national drug regulators -India has only one national regulator – the CDSCO
Now, in June 2009, at the 40th meeting of the Drugs Consultative Committee (DCC), it was decided that the Drug Controller General of India
13/n
(DCGI), who also heads the CDSCO, will take over the job of issuing COPPs from the state drug controllers for export. You know why?
Before this decision at the DCC, individual states were issuing such licenses. This caused the @WHO to tell the @CDSCO that because of
(DCGI), who also heads the CDSCO, will take over the job of issuing COPPs from the state drug controllers for export. You know why?
Before this decision at the DCC, individual states were issuing such licenses. This caused the @WHO to tell the @CDSCO that because of
14/n
the multitude of regulators and the variance in the process they each followed, it was becoming impossible for the WHO to manage this process. Therefore, it was decided that the CDSCO will take over this role as the National regulator.
the multitude of regulators and the variance in the process they each followed, it was becoming impossible for the WHO to manage this process. Therefore, it was decided that the CDSCO will take over this role as the National regulator.
17/n
The change above was challenged by the Karnataka Drugs and Pharmaceuticals Manufacturers Association before the Karnataka High Court which rejected the challenge and upheld the power of the DCGI/CDSCO to issue COPPs.
The change above was challenged by the Karnataka Drugs and Pharmaceuticals Manufacturers Association before the Karnataka High Court which rejected the challenge and upheld the power of the DCGI/CDSCO to issue COPPs.

18/n
The judgment records the following important submission of the CDSCO to the Court which was defending its takeover of the COPP function by the DCGI:
dineshthakur.com/wp-content/upl…
The judgment records the following important submission of the CDSCO to the Court which was defending its takeover of the COPP function by the DCGI:
dineshthakur.com/wp-content/upl…

19/n
Para 4 is basically the argument made by the CDSCO – it is clearly representing to the court that the WHO has recognised only the DCGI as the valid authority to grant COPPs (DCGI heads CDSCO).
Para 4 is basically the argument made by the CDSCO – it is clearly representing to the court that the WHO has recognised only the DCGI as the valid authority to grant COPPs (DCGI heads CDSCO).

20/n
The judgment also records the following interesting communications between the WHO and the DCGI wherein the former specifically congratulates the latter for putting in place a measure for centralizing the power to issue COPPs within the CDSCO.
The judgment also records the following interesting communications between the WHO and the DCGI wherein the former specifically congratulates the latter for putting in place a measure for centralizing the power to issue COPPs within the CDSCO.

22/n
In fact, as per the CDSCO guidelines reported in the press in 2009, a COPP certificate would be granted by zonal offices of the CDSCO on behalf of the DCGI, after central drug inspectors have inspected the manufacturing facility in question.
In fact, as per the CDSCO guidelines reported in the press in 2009, a COPP certificate would be granted by zonal offices of the CDSCO on behalf of the DCGI, after central drug inspectors have inspected the manufacturing facility in question.
23/n
However, as noted by the Karnataka High Court, there were some transitory difficulties in the CDSCO taking over the function and it appears that while the State Drug Controller continued to issue the COPP they could not do so without the central drug inspectors
However, as noted by the Karnataka High Court, there were some transitory difficulties in the CDSCO taking over the function and it appears that while the State Drug Controller continued to issue the COPP they could not do so without the central drug inspectors
24/n
from the CDSCO first conducting an inspection and submitting a report to the State Drug Controller.
A relevant extract from the judgment (as recorded from a letter by the then DCGI):
from the CDSCO first conducting an inspection and submitting a report to the State Drug Controller.
A relevant extract from the judgment (as recorded from a letter by the then DCGI):

25/n
These temporary transitory measures appear to have never been fixed. Why? This is a question only the CDSCO can answer. Perhaps some of you can ask the DCGI.
As a result, what the State Drug Controller does is purely ministerial since the basis of issuing the COPP is
These temporary transitory measures appear to have never been fixed. Why? This is a question only the CDSCO can answer. Perhaps some of you can ask the DCGI.
As a result, what the State Drug Controller does is purely ministerial since the basis of issuing the COPP is
26/n
the inspection by the central drug inspectors from the CDSCO.
Why else does name of Maiden Pharmaceuticals (the manufactured accused in the Gambian tragedy) appear on the CDSCO’s list of WHO-GMP Certified Manufacturing Units for COPP otherwise?
cdsco.gov.in/opencms/resour…
the inspection by the central drug inspectors from the CDSCO.
Why else does name of Maiden Pharmaceuticals (the manufactured accused in the Gambian tragedy) appear on the CDSCO’s list of WHO-GMP Certified Manufacturing Units for COPP otherwise?
cdsco.gov.in/opencms/resour…
28/n
The statement below ignores the fact that the basis of the COPP is a CDSCO inspection. Further if the above comments by the @CDSCO_INDIA_INF are true, what then of the assurances it made to the WHO that it was taking over the COPP program given
The statement below ignores the fact that the basis of the COPP is a CDSCO inspection. Further if the above comments by the @CDSCO_INDIA_INF are true, what then of the assurances it made to the WHO that it was taking over the COPP program given

29/n
its status as a national drug regulator? Is the WHO aware of the fact that the CDSCO is discounting all responsibility for the COPP program? @DrTedros
its status as a national drug regulator? Is the WHO aware of the fact that the CDSCO is discounting all responsibility for the COPP program? @DrTedros
30/n
One more thing, in their threatening letter to us because we said that once a manufacturing licence is granted the drug can be sold in India or exported, the CDSCO says: “No drug can be sold in the domestic market on the basis of a license which has been issued for
One more thing, in their threatening letter to us because we said that once a manufacturing licence is granted the drug can be sold in India or exported, the CDSCO says: “No drug can be sold in the domestic market on the basis of a license which has been issued for
31/n
the drug for export purpose only”.
There is no such requirement for an export licence in the Drugs & Cosmetics Act, 1940. This is precisely why the Ministry of Health proposed the Drugs & Cosmetics (Amendment) Bill 2007 and Drugs & Cosmetics (Amendment) Bill 2013 to
the drug for export purpose only”.
There is no such requirement for an export licence in the Drugs & Cosmetics Act, 1940. This is precisely why the Ministry of Health proposed the Drugs & Cosmetics (Amendment) Bill 2007 and Drugs & Cosmetics (Amendment) Bill 2013 to
32/n
specifically give the CDSCO control over exports. Both bills were subsequently withdrawn from the Parliament.
prsindia.org/billtrack/the-…
prsindia.org/files/bills_ac…
specifically give the CDSCO control over exports. Both bills were subsequently withdrawn from the Parliament.
prsindia.org/billtrack/the-…
prsindia.org/files/bills_ac…
33/n
In the law as is written now, there is no provision which requires pharma companies to procure a specific licence from any drug regulatory authority in India to export a drug.
Now that we have settled that argument, question is why such legal threats?
In the law as is written now, there is no provision which requires pharma companies to procure a specific licence from any drug regulatory authority in India to export a drug.
Now that we have settled that argument, question is why such legal threats?
35/n
Seriously?
Our response to the legal threat is available here:
dineshthakur.com/wp-content/upl…
Seriously?
Our response to the legal threat is available here:
dineshthakur.com/wp-content/upl…
36/n
Now, let me ask you something? Does this episode inspire confidence in the ability of the @CDSCO_INDIA_INF to ensure that we have a safe and effective drug supply chain?
What do you say?
Now, let me ask you something? Does this episode inspire confidence in the ability of the @CDSCO_INDIA_INF to ensure that we have a safe and effective drug supply chain?
What do you say?
37/n
If you have stayed with me for this long and are worried about the quality of our drug supply, I urge you to please read our book “The Truth Pill – The Myth of Drug Regulation in India” #truthpill
truthpill.in
If you have stayed with me for this long and are worried about the quality of our drug supply, I urge you to please read our book “The Truth Pill – The Myth of Drug Regulation in India” #truthpill
truthpill.in

One last thing, BTW.
The Gambian Law says this:
mca.gm/medicines-and-…
“34. Certificate of Imported Medicines: Where a medicine or related product is imported as a finished product, an application for the registration of that article shall be accompanied by a certificate of
The Gambian Law says this:
mca.gm/medicines-and-…
“34. Certificate of Imported Medicines: Where a medicine or related product is imported as a finished product, an application for the registration of that article shall be accompanied by a certificate of
analysis or certificate of pharmaceutical product for medicines issued by the competent regulatory agency of the exporting country”
FWIW
FWIW
• • •
Missing some Tweet in this thread? You can try to
force a refresh