
Let's talk about bone marrow fibrosis in myelofibrosis.
When we hear fibrosis, we think lung (IPF) or liver (cirrhosis), devastating conditions.
The beauty about marrow fibrosis: it's reversible with allogeneic BMT.
Let's start🧵with new #ASH22 abstracts
#mpnsm #MedTwitter /19
When we hear fibrosis, we think lung (IPF) or liver (cirrhosis), devastating conditions.
The beauty about marrow fibrosis: it's reversible with allogeneic BMT.
Let's start🧵with new #ASH22 abstracts
#mpnsm #MedTwitter /19
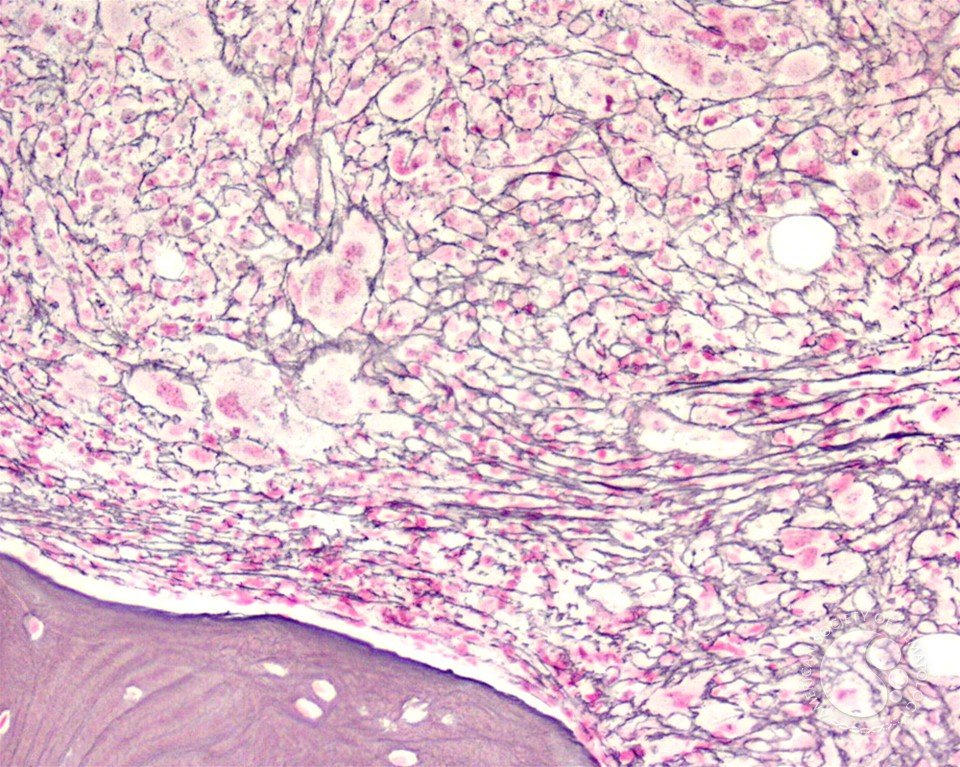
Bone marrow fibrosis (BMF) is characterized by the increased deposition of reticulin fibers and in some cases collagen fibers. Scoring of BMF is primarily dependent on manual grading by the hematopathologist based on the density and type of fibrosis. 1/19 

Besides myelofibrosis, there are several hematologic and non-hematologic disorders that are associated with increased BMF that differ in composition (either reticulin-only or reticulin plus collagen). 2/19 

Myelofibrosis (MF) is a Philadelphia-negative chronic myeloproliferative neoplasm, characterized by variable degrees of BMF, abnormal proliferation of hematopoietic progenitor cells (HPC), cytopenias, splenomegaly, increased risk of thrombosis and blast transformation. 3/19 

History part 2: In 1951, thanks to William Dameshek, it was shown that primary MF shares clinical and pathological features with essential thrombocythemia and polycythemia vera, which also can progress to MF: recently confirmed by discovery of a common mutational background.4/19 

History part 2: Since 2005, we know that up to 90% of patients harbor at least 1 of so-called “driver” mutations: V617F in JAK2 gene, W515L/K in MPL gene, and 52 bp deletion (type 1 mutation) or 5 bp insertion (type 2 mutation) in CALR gene.5/19 

Back to fibrosis.
Although the exact pathogenesis of MF is not fully understood, let's quickly go through potential drivers and treatment options for BMF.
Drivers:
malignant clone
inflammation
cytokines
cellular interaction
genetics
6/19
Although the exact pathogenesis of MF is not fully understood, let's quickly go through potential drivers and treatment options for BMF.
Drivers:
malignant clone
inflammation
cytokines
cellular interaction
genetics
6/19
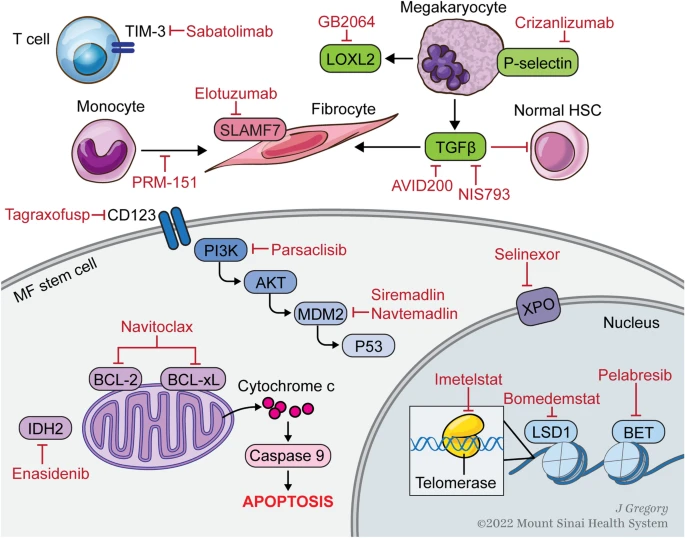
Goals of MF treatment can be divided into 2 categories: clonal eradication and treatments targeting various signaling pathways and mediators implicated in BMF.
Allogeneic BMT is the only curative treatment, eradicates the malignant HSC clone, leading to reversal of BMF👇
7/19
Allogeneic BMT is the only curative treatment, eradicates the malignant HSC clone, leading to reversal of BMF👇
7/19

Inflammation:
could be involved in determining genetic damage to HSC, through oxidative damage to DNA by reactive oxygen species (ROS).
TGF-β1/miR-382-5p/SOD2 axis deregulation linked to ROS overproduction.
Galunisertib potentially reduces oxidative stress induced by TGF-β1. 8/19
could be involved in determining genetic damage to HSC, through oxidative damage to DNA by reactive oxygen species (ROS).
TGF-β1/miR-382-5p/SOD2 axis deregulation linked to ROS overproduction.
Galunisertib potentially reduces oxidative stress induced by TGF-β1. 8/19

Inflammation:
provides a continuous stimulus for Gli1+ myofibroblasts to nurture the process of BMF. Genetic ablation of Gli1+ cells abolished MF and rescues BM failure; and pharmacological targeting of Gli1+ cells with GANT61 ameliorated MF. 9/19
provides a continuous stimulus for Gli1+ myofibroblasts to nurture the process of BMF. Genetic ablation of Gli1+ cells abolished MF and rescues BM failure; and pharmacological targeting of Gli1+ cells with GANT61 ameliorated MF. 9/19

Cytokines:
TGF-β signaling plays a central role in the pathogenesis of BMF in MF. Luspatercept is a TGFβ trap that consists of the extracellular domain of the activin receptor II B fused to the Fc domain of human IgG1. Early results showed Hb increases but no data on BMF. 10/19
TGF-β signaling plays a central role in the pathogenesis of BMF in MF. Luspatercept is a TGFβ trap that consists of the extracellular domain of the activin receptor II B fused to the Fc domain of human IgG1. Early results showed Hb increases but no data on BMF. 10/19

Interactions:
Leptin receptor-expressing stromal cells can differentiate into myofibroblasts. Imatinib or conditional deletion of platelet-derived growth factor receptor a (Pdgfra) from Lepr+ stromal cells suppressed their expansion and ameliorated bone marrow fibrosis. 11/19
Leptin receptor-expressing stromal cells can differentiate into myofibroblasts. Imatinib or conditional deletion of platelet-derived growth factor receptor a (Pdgfra) from Lepr+ stromal cells suppressed their expansion and ameliorated bone marrow fibrosis. 11/19

Genetics:
big topic, will make it concise and focus on new drugs with upcoming fresh and (in part) exciting results at #ASH22 #mpnsm 12/19
big topic, will make it concise and focus on new drugs with upcoming fresh and (in part) exciting results at #ASH22 #mpnsm 12/19

Telomerase inhibition:
Imetelstat is a 13-mer lipid-conjugated oligonucleotide that complimentary binds the template region of telomerase. Despite only modest spleen responses, there was a reduction in BMF in 15 evaluable patients (40.5%).
->very limited evidence. 13/19
Imetelstat is a 13-mer lipid-conjugated oligonucleotide that complimentary binds the template region of telomerase. Despite only modest spleen responses, there was a reduction in BMF in 15 evaluable patients (40.5%).
->very limited evidence. 13/19
MDM2:
a key negative regulator of the TP53 pathway, inhibiting TP53 transcription, facilitating export of TP53 from the nucleus, and promoting the degradation of TP53. MDM2i Navtemdalin showed improvement of at least 1 grade in BMF in 1/4, now explored in phase 2/3 study. 14/19
a key negative regulator of the TP53 pathway, inhibiting TP53 transcription, facilitating export of TP53 from the nucleus, and promoting the degradation of TP53. MDM2i Navtemdalin showed improvement of at least 1 grade in BMF in 1/4, now explored in phase 2/3 study. 14/19
HDM2:
Siremadlin, potent and selective HDM2 inhibitor, restores p53-mediated apoptosis. Results on spleen volume 👇
Impact on BMF to be shown. #ASH22
ash.confex.com/ash/2022/webpr… 15/19
Siremadlin, potent and selective HDM2 inhibitor, restores p53-mediated apoptosis. Results on spleen volume 👇
Impact on BMF to be shown. #ASH22
ash.confex.com/ash/2022/webpr… 15/19

BET:
facilitates transcription of genes regulated by NF-κB, c-Myc, and TGF-β. BETi pelabresib showed BMF reduction in 16/57 patients; 1/2 maintained through week 48 or beyond. #ASH22
ash.confex.com/ash/2022/webpr… 16/19
facilitates transcription of genes regulated by NF-κB, c-Myc, and TGF-β. BETi pelabresib showed BMF reduction in 16/57 patients; 1/2 maintained through week 48 or beyond. #ASH22
ash.confex.com/ash/2022/webpr… 16/19

BCL:
JAK-STAT pathway constitutive activation increases BCL-2 and BCL-xL expression. Navitoclax (BCLi) and ruxolitinib showed BMF reduction in 26/32 (81%) patients. Complete resolution of BMF in 2/9 patients, baseline was grade 2 and 3. #ASH22 ash.confex.com/ash/2022/webpr… 17/19
JAK-STAT pathway constitutive activation increases BCL-2 and BCL-xL expression. Navitoclax (BCLi) and ruxolitinib showed BMF reduction in 26/32 (81%) patients. Complete resolution of BMF in 2/9 patients, baseline was grade 2 and 3. #ASH22 ash.confex.com/ash/2022/webpr… 17/19

Immunotherapy:
a highly potent monoclonal antibody that binds to mutated CALR and inhibits oncogenesis in cells expressing CALR, demonstrating that this moAbnormalizes TPO-R signaling in patient-derived HSCs and in a in vivo model. #ASH22 ash.confex.com/ash/2022/webpr… 18/19
a highly potent monoclonal antibody that binds to mutated CALR and inhibits oncogenesis in cells expressing CALR, demonstrating that this moAbnormalizes TPO-R signaling in patient-derived HSCs and in a in vivo model. #ASH22 ash.confex.com/ash/2022/webpr… 18/19
Finally, is BMF reduction even relevant?
1. BMT does not care about BMF grade->no impact on post-BMT outcome.
2. Anemia improvement was not linked with BMF changes->question the use of BMF assessment at as a surrogate for clinical benefit. #ASH22 ash.confex.com/ash/2022/webpr… 19/19
1. BMT does not care about BMF grade->no impact on post-BMT outcome.
2. Anemia improvement was not linked with BMF changes->question the use of BMF assessment at as a surrogate for clinical benefit. #ASH22 ash.confex.com/ash/2022/webpr… 19/19
In conclusion, we did not fully understand the role of BMF yet. Allo BMT as only complete disease modifier, while new drugs seem to be associated with BMF reduction. Whether this is only an epi-phenomenon of is unclear. So, let's be careful to use BMF as endpoint.
Helpful sources:
DOI: 10.1038/ncb3530
link.springer.com/article/10.100…
sciencedirect.com/science/articl…
f1000research.com/articles/5-700…
ashpublications.org/blood/article/…
DOI: 10.1038/ncb3530
link.springer.com/article/10.100…
sciencedirect.com/science/articl…
f1000research.com/articles/5-700…
ashpublications.org/blood/article/…
• • •
Missing some Tweet in this thread? You can try to
force a refresh






