You read a lot about "novel" drugs in myelofibrosis.
But you need to know how to walk before you fly.
JAK inhibitors were the first to revolutionize myelofibrosis treatment.
Let's recap what they are & what we still dont know.
🧵with #ASH22 abstracts
#MedTwitter #mpnsm
But you need to know how to walk before you fly.
JAK inhibitors were the first to revolutionize myelofibrosis treatment.
Let's recap what they are & what we still dont know.
🧵with #ASH22 abstracts
#MedTwitter #mpnsm

Janus kinase:
-intracellular, non-receptor tyrosine kinases that transduce cytokine-mediated signals via the JAK-STAT pathway
-name taken from the 2-faced Roman god of beginnings, endings and duality, because JAKs possess near-identical phosphate-transferring domains 2/17
-intracellular, non-receptor tyrosine kinases that transduce cytokine-mediated signals via the JAK-STAT pathway
-name taken from the 2-faced Roman god of beginnings, endings and duality, because JAKs possess near-identical phosphate-transferring domains 2/17

There are currently 4 JAK inhibitors for MF:
ruxolitinib
fedratinib
pacritinib
momelotinib
Let's quickly present them, followed up by #ASH22 abstracts. 3/17
ruxolitinib
fedratinib
pacritinib
momelotinib
Let's quickly present them, followed up by #ASH22 abstracts. 3/17

Ruxolitinib:
-selective for JAK1/2
-approved '11
-COMFORT-1/2 trials: in higher risk patients with platelets ≥100->spleen reduction in ~4/10 and ~5/10 experienced a ≥50% improvement in total symptom score (follow-up 24 weeks)
-no survival results
-cornerstone for MF 4/17
-selective for JAK1/2
-approved '11
-COMFORT-1/2 trials: in higher risk patients with platelets ≥100->spleen reduction in ~4/10 and ~5/10 experienced a ≥50% improvement in total symptom score (follow-up 24 weeks)
-no survival results
-cornerstone for MF 4/17

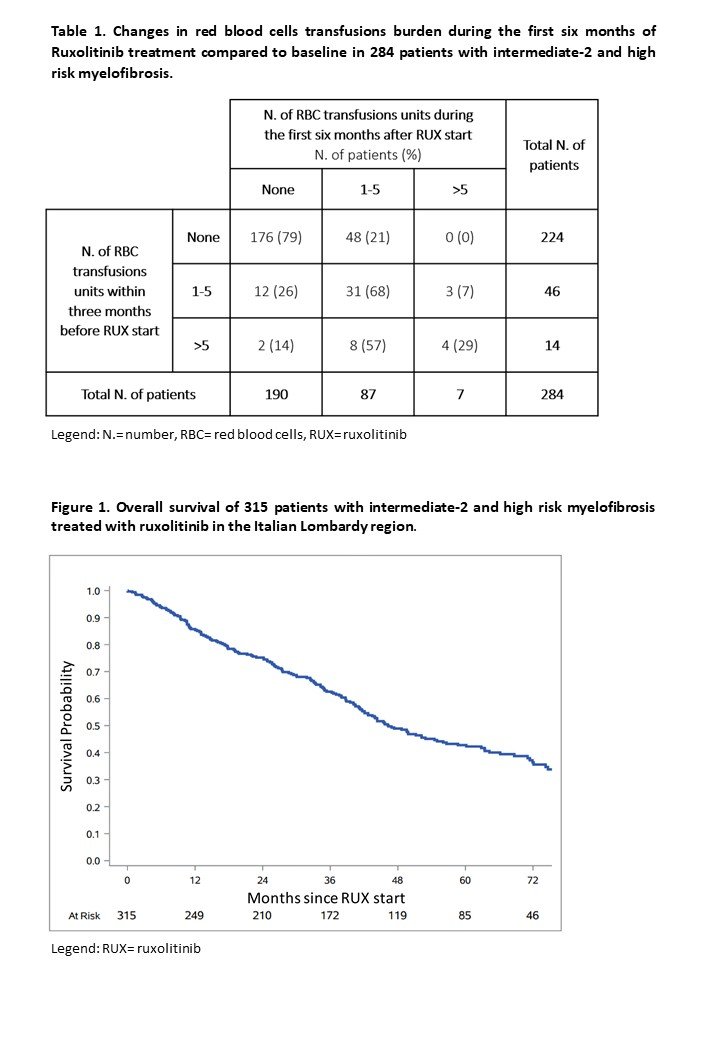
Ruxo #ASH22:
-higher risk MF in 🇮🇹 Lombardy
-median survival was 46 months, ruxo failure ~5/10 patients (median time to failure 2years)
-AlloBMT in only 1/10
-infections, bleeding events and thrombosis
-evolution into blast-phaseMF or MDS in 2/10 ash.confex.com/ash/2022/webpr… 5/17
-higher risk MF in 🇮🇹 Lombardy
-median survival was 46 months, ruxo failure ~5/10 patients (median time to failure 2years)
-AlloBMT in only 1/10
-infections, bleeding events and thrombosis
-evolution into blast-phaseMF or MDS in 2/10 ash.confex.com/ash/2022/webpr… 5/17
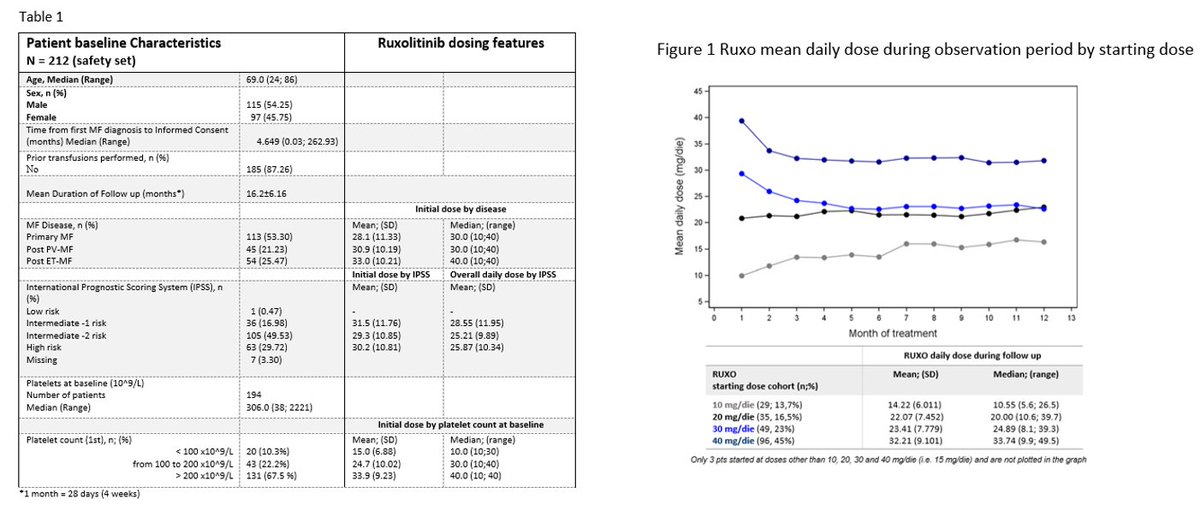
Ruxo #ASH22:
-dosing pattern in 🇮🇹
-suboptimal use in practice vs approved indication
-1/4 with platelets >200 not assigned to the recommended starting dose (20 mg bid)
-most received dose adjustment during treatment
->we still dont know how to dose!ash.confex.com/ash/2022/webpr… 6/17
-dosing pattern in 🇮🇹
-suboptimal use in practice vs approved indication
-1/4 with platelets >200 not assigned to the recommended starting dose (20 mg bid)
-most received dose adjustment during treatment
->we still dont know how to dose!ash.confex.com/ash/2022/webpr… 6/17
Ruxo #ASH22:
-subsequent malignancies
-report of patients diagnosed with nonmelanoma skin cancer
-aggressive, high recurrence rate, metastatic spread and mortality
-unclear whether due to immunosuppression or ?? ash.confex.com/ash/2022/webpr… 7/17
-subsequent malignancies
-report of patients diagnosed with nonmelanoma skin cancer
-aggressive, high recurrence rate, metastatic spread and mortality
-unclear whether due to immunosuppression or ?? ash.confex.com/ash/2022/webpr… 7/17

Fedratinib:
-JAK2 inhibitor
-efficacy and heme tox similar to ruxo
-but initially halted due to Wernicke encephalopathy
-also severe GI toxicity through FLT3 inhibitory effect
-JAKARTA trial: 400 mg daily, 3/10 with response
->again, no survival results 8/17
-JAK2 inhibitor
-efficacy and heme tox similar to ruxo
-but initially halted due to Wernicke encephalopathy
-also severe GI toxicity through FLT3 inhibitory effect
-JAKARTA trial: 400 mg daily, 3/10 with response
->again, no survival results 8/17

Fedra #ASH22:
-FREEDOM trial, recruitment problem due to COVID
-38 patients
-25 discontinued
-1/4 met primary endpoint of ≥35% spleen volume reduction
-1/2 showed ≥50% reduction in symptom score
-nausea & diarrhea in 4/10
-no encephalopathy ash.confex.com/ash/2022/webpr… 9/17
-FREEDOM trial, recruitment problem due to COVID
-38 patients
-25 discontinued
-1/4 met primary endpoint of ≥35% spleen volume reduction
-1/2 showed ≥50% reduction in symptom score
-nausea & diarrhea in 4/10
-no encephalopathy ash.confex.com/ash/2022/webpr… 9/17

Pacritinib:
-JAK2 but also 1 & 3, ACVR1, IRAK1, FLT3
-approved 2022 for MF with platelet counts <50
-potent anti-proliferative effects on myeloid and lymphoid cell lineages driven by mutant or wild-type kinases, limited myelo- and immunosuppressive activity👇 10/17
-JAK2 but also 1 & 3, ACVR1, IRAK1, FLT3
-approved 2022 for MF with platelet counts <50
-potent anti-proliferative effects on myeloid and lymphoid cell lineages driven by mutant or wild-type kinases, limited myelo- and immunosuppressive activity👇 10/17

Pac #ASH22:
-four-fold higher potency for ACVR1 vs momelotinib -associated with improvement in transfusion requirement
-effect size maintained among patients who had not received ruxo within 30 days prior to treatment with pac ash.confex.com/ash/2022/webpr… 11/17
-four-fold higher potency for ACVR1 vs momelotinib -associated with improvement in transfusion requirement
-effect size maintained among patients who had not received ruxo within 30 days prior to treatment with pac ash.confex.com/ash/2022/webpr… 11/17

Momelotinib:
-inhibitor of JAK1, JAK2, ACVR1, and FLT3
-previous trials failed to demonstrate the superiority of mome vs best available therapy (incl ruxo) in terms of spleen reduction
-now phase 3 trial (vs danazol=no MF specific treatment) in ruxo treated patients👇 12/17
-inhibitor of JAK1, JAK2, ACVR1, and FLT3
-previous trials failed to demonstrate the superiority of mome vs best available therapy (incl ruxo) in terms of spleen reduction
-now phase 3 trial (vs danazol=no MF specific treatment) in ruxo treated patients👇 12/17
Mome #ASH22:
-previous results: symptom response 1/4 in mome vs 1/10 in dana, zero transfusions 1/3 vs 1/7
-update: median follow-up for survival 51 weeks
-Hazard ratio 0.51, p=0.07->looses impact after cross-over (hazard ratio 0.95) ash.confex.com/ash/2022/webpr… 13/17
-previous results: symptom response 1/4 in mome vs 1/10 in dana, zero transfusions 1/3 vs 1/7
-update: median follow-up for survival 51 weeks
-Hazard ratio 0.51, p=0.07->looses impact after cross-over (hazard ratio 0.95) ash.confex.com/ash/2022/webpr… 13/17

Profiling of JAKi #ASH22:
-all 4 inhibitors show affinity in suppressing JAK-STAT but with varying degrees of alteration of transcriptional, proteomic, and metabolic profiles
-real understanding of response and potential synergism limited ash.confex.com/ash/2022/webpr… 14/17
-all 4 inhibitors show affinity in suppressing JAK-STAT but with varying degrees of alteration of transcriptional, proteomic, and metabolic profiles
-real understanding of response and potential synergism limited ash.confex.com/ash/2022/webpr… 14/17

JAKi failure #ASH22:
-patients failure of first line JAK inihibition managed with alloBMT (n=41) or best available therapy (n=47) showed better outcome for alloBMT
-BAT (fedra, pac, mome, ruxo continuation)
-follow-up short ash.confex.com/ash/2022/webpr… 15/17
-patients failure of first line JAK inihibition managed with alloBMT (n=41) or best available therapy (n=47) showed better outcome for alloBMT
-BAT (fedra, pac, mome, ruxo continuation)
-follow-up short ash.confex.com/ash/2022/webpr… 15/17

In that respect, do NOT wait for JAKi failure!
Ruxo before alloBMT does not negatively impact outcome and patients with ongoing spleen response at time of alloBMT show best outcome.
Refer early for alloBMT evaluation, assess response and let's improve lives! #mpnsm 16/17
Ruxo before alloBMT does not negatively impact outcome and patients with ongoing spleen response at time of alloBMT show best outcome.
Refer early for alloBMT evaluation, assess response and let's improve lives! #mpnsm 16/17

In conclusion, we don't really know whether ruxolitinib actually improves survival. Spleen response rates generally are <50% for all JAKi.
Before we design delicate surrogate endpoints (association with survival not proven), aim higher🙏
Treatment sequence is key in MF! 17/17
Before we design delicate surrogate endpoints (association with survival not proven), aim higher🙏
Treatment sequence is key in MF! 17/17
Helpful sources:
link.springer.com/article/10.100…
tandfonline.com/doi/full/10.10…
pubmed.ncbi.nlm.nih.gov/34023851/
ncbi.nlm.nih.gov/pmc/articles/P…
link.springer.com/article/10.100…
tandfonline.com/doi/full/10.10…
pubmed.ncbi.nlm.nih.gov/34023851/
ncbi.nlm.nih.gov/pmc/articles/P…
• • •
Missing some Tweet in this thread? You can try to
force a refresh