Here's a physiology case that *everyone* who touches a ventilator needs to understand:
A 60 yo woman is intubated for hypoxemia from multifocal pneumonia.
She has a SpO2 of 89% on PEEP +12 and 100% FiO2.
PEEP is increased to +16 & her SpO2 drops to 80%!
What happened?
1/
A 60 yo woman is intubated for hypoxemia from multifocal pneumonia.
She has a SpO2 of 89% on PEEP +12 and 100% FiO2.
PEEP is increased to +16 & her SpO2 drops to 80%!
What happened?
1/
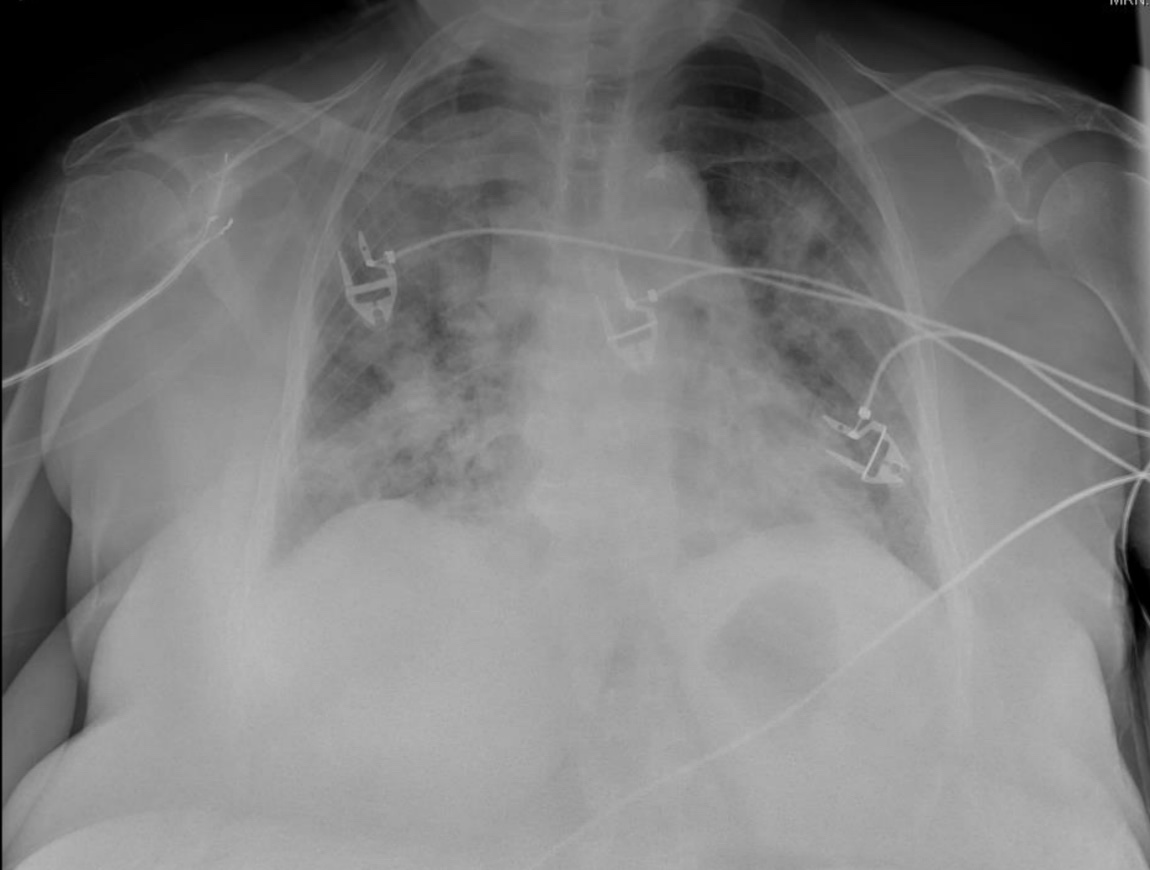
Before we get into the answer, let's make it interesting with some multiple choice.
Which mechanism(s) could cause worsening hypoxemia with increasing PEEP?
2/
Which mechanism(s) could cause worsening hypoxemia with increasing PEEP?
2/
Wow! 6000 votes! Im amazed by how many people share my love for 🫁 physiology!
I’ll post the answer tomorrow. If you can’t wait there’s a complete explanation on #MedMastodon.
(Btw I’ll posting answers sooner along with bonus content there from now on)
med-mastodon.com/@nick/10949932…
I’ll post the answer tomorrow. If you can’t wait there’s a complete explanation on #MedMastodon.
(Btw I’ll posting answers sooner along with bonus content there from now on)
med-mastodon.com/@nick/10949932…
The answer is ALL of the above!
PEEP can cause hypoxemia due to intra-pulmonary (everyone) and intracardiac shunt (some people), & by decreasing cardiac output (occasionally)!
But why? To answer, we need to understand what PEEP is & what effects it has on the heart & lung.
3/
PEEP can cause hypoxemia due to intra-pulmonary (everyone) and intracardiac shunt (some people), & by decreasing cardiac output (occasionally)!
But why? To answer, we need to understand what PEEP is & what effects it has on the heart & lung.
3/

Positive end expiratory pressure (PEEP) is the pressure above atmospheric applied in between breaths while on a ventilator.
PEEP is beneficial for 2 reasons:
1️⃣ it recruits collapsed lung (see👇)
2️⃣ higher P drives more O2 into the blood (Henry's law)
4/
PEEP is beneficial for 2 reasons:
1️⃣ it recruits collapsed lung (see👇)
2️⃣ higher P drives more O2 into the blood (Henry's law)
https://twitter.com/nickmmark/status/1579951658012151811?s=21
4/
As an aside, the benefits of PEEP were discovered *accidentally*.
In 1967, two doctors spotted an unfamiliar knob on the ventilator labeled "expiratory retard" & - not knowing what it did - decided to give it a turn.
Nowadays we call that knob PEEP!
pubmed.ncbi.nlm.nih.gov/28731363/
5/

In 1967, two doctors spotted an unfamiliar knob on the ventilator labeled "expiratory retard" & - not knowing what it did - decided to give it a turn.
Nowadays we call that knob PEEP!
pubmed.ncbi.nlm.nih.gov/28731363/
5/


So we understand why PEEP can help, but why can it be *harmful*?
We need to understand the relationship between lung volumes & blood flow.
Let's take a closer look. With an electron microscope we can see that alveoli are surrounded by a dense network of blood vessels.
6/

We need to understand the relationship between lung volumes & blood flow.
Let's take a closer look. With an electron microscope we can see that alveoli are surrounded by a dense network of blood vessels.
6/


There's a dynamic relationship between alveoli inflation & blood flow.
As the alveoli become more inflated, blood flow through these dense intra-alveolar vessels decreases. This increases the pulmonary vascular resistance (PVR).
7/
As the alveoli become more inflated, blood flow through these dense intra-alveolar vessels decreases. This increases the pulmonary vascular resistance (PVR).
7/

PVR is lowest at Functional residual capacity (where normal tidal breathing occurs). PVR increases with both lower or higher lung volumes.
(btw, this is an important fact to remember when managing RV failure & PA hypertension too)
8/

(btw, this is an important fact to remember when managing RV failure & PA hypertension too)
8/


Excessive PEEP overdistends alveoli & decreases blood flow through the intra-alveolar vessels responsible for gas exchange.
It also increases blood flow in the extra-alveolar blood vessels that don't participate in gas exchange.
This causes intra-pulmonary shunt & hypoxemia!
8/
It also increases blood flow in the extra-alveolar blood vessels that don't participate in gas exchange.
This causes intra-pulmonary shunt & hypoxemia!
8/

Another factor to consider is that PEEP may be uniform, especially if different areas have different compliance.
Areas of the lung affected by pneumonia may not be recruitable, but normal areas may be overdistended by too much PEEP. This too worsens intra-pulmonary shunt.
9/
Areas of the lung affected by pneumonia may not be recruitable, but normal areas may be overdistended by too much PEEP. This too worsens intra-pulmonary shunt.
9/

Now that we understand how PEEP effects the lungs, we also must consider how PEEP effects the heart.
We've already talked about how larger volumes can increase PVR. This increases RV afterload & right sided pressures.
For the 25% of the population with a PFO, this matters!
10/
We've already talked about how larger volumes can increase PVR. This increases RV afterload & right sided pressures.
For the 25% of the population with a PFO, this matters!
10/

One study found that the two biggest predictors of right to left shunt through a PFO were the degree of RV dilation & higher plateau pressures. Excessive PEEP can increase both!
ncbi.nlm.nih.gov/pmc/articles/P…
Be suspicious if a small change in PEEP causes a big drop in SpO2.
11/
ncbi.nlm.nih.gov/pmc/articles/P…
Be suspicious if a small change in PEEP causes a big drop in SpO2.
11/
Finally, let's consider the effects of PEEP on cardiac output.
PEEP decreases venous return because of increased intrathoracic pressure. Depending on volume status a decrease in preload *usually* decreases CO.
(great explanation @derangedphys)
ncbi.nlm.nih.gov/pmc/articles/P…
12/

PEEP decreases venous return because of increased intrathoracic pressure. Depending on volume status a decrease in preload *usually* decreases CO.
(great explanation @derangedphys)
ncbi.nlm.nih.gov/pmc/articles/P…
12/


Decreasing CO has many effects (hypotension, reflex tachycardia, decreased UOP, etc).
But why can low CO worsen hypoxemia?
Recall that low CO drops SvO2. If your SvO2 drops enough it will worsen hypoxemia. This is the SIXTH cause of hypoxemia.
See my ICU OnePager for more
13/
But why can low CO worsen hypoxemia?
Recall that low CO drops SvO2. If your SvO2 drops enough it will worsen hypoxemia. This is the SIXTH cause of hypoxemia.
See my ICU OnePager for more
13/

So now that you're experts in the physiology of PEEP, let's put this all together.
There are 2 mechanisms where PEEP can improve oxygenation:
1️⃣ alveolar recruitment
2️⃣ higher mean airway pressure (Henry's law)
14/
There are 2 mechanisms where PEEP can improve oxygenation:
1️⃣ alveolar recruitment
2️⃣ higher mean airway pressure (Henry's law)
14/
And 3 mxns where PEEP can worsen hypoxemia:
1️⃣ intra-pulmonary shunt (overdistension of alveoli & shunt into extra-alveolar vessels)
2️⃣ intra-cardiac shunt (via a PFO; in the 25-30% of people who have one)
3️⃣ decreased CO (particularly in people with low CO at baseline)
15/
1️⃣ intra-pulmonary shunt (overdistension of alveoli & shunt into extra-alveolar vessels)
2️⃣ intra-cardiac shunt (via a PFO; in the 25-30% of people who have one)
3️⃣ decreased CO (particularly in people with low CO at baseline)
15/
The best (and fastest) way to evaluate for 2️⃣ & 3️⃣ is with point of care ultrasound.
Looking for bubbles in the LA after agitated saline can help spot a PFO. Measuring LVOT VTI at different PEEPs can be very helpful in titrating. Remember to r/o PTX too!
15/
Looking for bubbles in the LA after agitated saline can help spot a PFO. Measuring LVOT VTI at different PEEPs can be very helpful in titrating. Remember to r/o PTX too!
15/
It's very helpful to compare measurements at different PEEP values to help find the "sweet spot" for oxygenation, compliance, and cardiac output. Something like this (though maybe with a column for LVOT VTI):
Source: advances.massgeneral.org/pulmonary/arti…
16/
Source: advances.massgeneral.org/pulmonary/arti…
16/

I hope you've enjoyed this thread.
To learn more about this important topic, including a really nice deep dive into the physiology, I *highly* recommend this paper by @basakcoruhUW & Andy Luks.
atsjournals.org/doi/pdf/10.151….
17/17
To learn more about this important topic, including a really nice deep dive into the physiology, I *highly* recommend this paper by @basakcoruhUW & Andy Luks.
atsjournals.org/doi/pdf/10.151….
17/17
• • •
Missing some Tweet in this thread? You can try to
force a refresh