
The #Activ6 trial ivermectin 600μg/kg is a scientific fraud. medrxiv.org/content/10.110…
Here is how they justify dosage and duration: "a possible anti-viral activity with increasing dose." citing Krolewiecki et al. study.
1/n
Here is how they justify dosage and duration: "a possible anti-viral activity with increasing dose." citing Krolewiecki et al. study.
1/n

If we look at the protocol of the activ6 trial published as a supplement of the 400μg/kg arm published in the JAMA: jamanetwork.com/journals/jama/…, here is the rationale for selection of dose (600μg/kg).
2/n
2/n

Reference 66 is Krolewiecki et al. study. 1rst problem: Krolewiecki study doesn't contain a 300μg/kg as they claim here. What is right is "The anti-viral activity was identified in the subgroup of patients on ivermectin with higher mean plasma concentrations."
3/n
3/n
Now if we look at the Krolewiecki study: sciencedirect.com/science/articl… we see that there is a single dosage of IVM: 600μg/kg during 5 days.
4/n
4/n
No difference in viral load is seen in the first place, but the authors identify 2 sub-groups according to mean plasma IVM concentration (+/-160ng/mL) and noticed an antiviral effect in the high concentration group:
5/n
5/n

"Mean IVM plasma concentration levels also showed a positive correlation with viral decay rate".
6/n
6/n
The authors stressed that "diet is a key variable affecting oral bioavailability of IVM, with increased plasma concentrations achieved with fed state" and identify it as a potential cause of variation of mean plasma concentration in their trial.
7/n

7/n
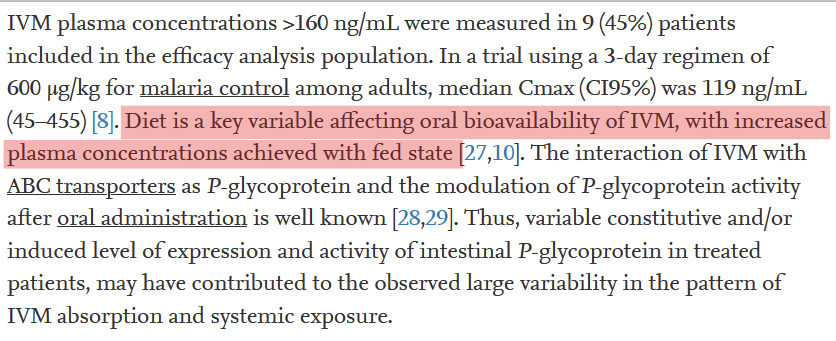

The conclusion for the rationale for selection of dose from Krolewiecki study is that not only dosage is important, but prescription with or after fat meal is crucial to see antiviral effect.
8/n
8/n
Now if we return to #activ6 protocol, we see that:
1/ They misinterpret Krolewiecki study by acknowledging only dosage parameter: "These data argue for the use of higher dosing regimens in clinical trials" when the prescription on fed state is crucial.
9/n
1/ They misinterpret Krolewiecki study by acknowledging only dosage parameter: "These data argue for the use of higher dosing regimens in clinical trials" when the prescription on fed state is crucial.
9/n

2/ They especially specify that drug should be taken on an empty stomach, knowing from Krolewiecki et al. that this is the key factor that lead to low levels of mean IVM plasma concentration, thus the less chance of seeing an antiviral effect.
10/n
10/n

Conclusion: the rationale for dosing of #Activ6 trial ivermectin 600μg/kg is based on potential anti-viral effect, but the whole trial is not designed to evaluate the anti-viral effect.
11/n
11/n
In addition to the low mean plasma concentration targeted, the key factor of an anti-viral drug should start the treatment early (<5 days after symptoms onset or even better <3 days).
12/n
12/n
See the paxlovid trial for an example of how an antiviral should be tested in clinical trials: nejm.org/doi/full/10.10…: early treatment, viral load measures at regular intervals, relevant inclusion criteria and outcome.
13/n
13/n
Now, I hope @NEJM, @JAMA_current or @TheLancet will refuse to publish this fraudulent trial designed to fail, and I hope reviewers will request a clear explanation of the "empty stomach" choice of the investigators.
14/n
14/n
It should be clarified that the best protocol, based on scientific knowledge, was knowingly discarded.
FYI @SabinehazanMD , @PierreKory , @lawrie_dr , @alexandrosM .
15/15
FYI @SabinehazanMD , @PierreKory , @lawrie_dr , @alexandrosM .
15/15
• • •
Missing some Tweet in this thread? You can try to
force a refresh




