
Very excited to share our new study on determinants of resistance to anti-CD19 CAR T-cells (CART19) in large B-cell lymphomas (LBCLs)! Out today in @Cancer_Cell /1
cell.com/cancer-cell/fu…
cell.com/cancer-cell/fu…
A fantastic collaboration from @StanfordMed and @StanfordCancer led by Brian Sworder, David Kurtz, @StefanAlig, and Matthew Frank with David Miklos & @max_diehn to help improve outcomes following CAR T-cell therapy in lymphomas & beyond /2
We serially profiled plasma & tumor samples from 138 r/rLBCL patients across 2 independent cohorts treated w/ SOC axicabtagene ciloleucel (#axicel #yescarta) @StanfordBMT_CT /3 

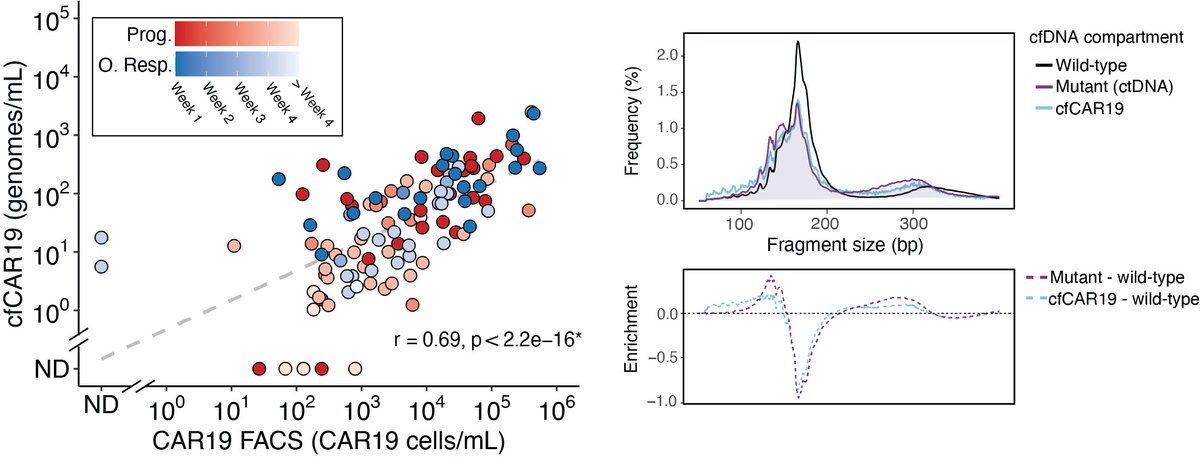
To study both tumor & effector mediated resistance determinants, we developed a method (STEP) to simultaneously profile #ctDNA, cell-free CAR T-cell retroviral fragments (cfCAR), and cell-free T-cell receptor (cfTCR) rearrangements from a plasma. /4 

ctDNA levels were followed serially, and patients with higher pretreatment ctDNA or inadequate ctDNA responses had inferior outcomes. /5 

CAR T-cell cfDNA (cfCAR19) levels correlated with #CARFACS measurements and cfCAR19 molecules demonstrated a fragment length profile similar to that of ctDNA, indicating that #cfDNA can be used to both quantify and profile CAR T-cell biodistribution. /6 
Alterations in multiple classes of genes were associated with resistance, including B-cell identity (PAX5 and IRF8), immune checkpoints (CD274/PD-L1) and those impacting the microenvironment (TMEM30A). /7 

Tumors with high levels of infiltrating CAR T-cells at the time of relapse also had high cfCAR19 levels, and demonstrated distinct tumor microenvironmental and gene expression signatures. /8 

A multivariable model (#STEP Score) incorporating both tumor (week 4 ctDNA level) and T effector mediated (week 1 cfCAR19 level) factors that was trained and validated across the two cohorts was predictive of both event-free survival and overall-survival. /9 

CAR T-cell resistance in LBCL is dynamic and multifactorial, with contributions from both target tumor-cell and effector T-cell mediated factors. /10
We're indebted to all of our co-authors and our collaborations with @YasoNatkunam @StanfordBMT_CT @Majzner_Lab @MackallLab @jayspiegel25 John Baird Michael Khodadoust @MDAndersonNews @DrJasonWestin @UniklinikEssen Uli Dührsen Andreas Hüttmann Christine Hanoun. /11
12. We are so grateful for the patients and their families for participating in our studies. And are honored to have had the support of @NIH @NCI @LLSusa @SU2C @lymphoma @Ludwig_Cancer @DamonRunyon @ConquerCancerFd @SCIDirector @UpliftingAth @SusanWojcicki . /12 EOT
• • •
Missing some Tweet in this thread? You can try to
force a refresh




