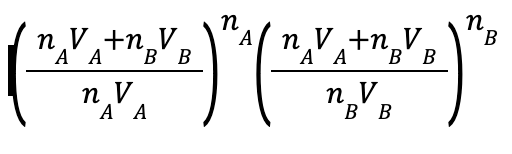#thermofact
What does the Boltzmann constant mean?
1/ Temperature has many units, among which are Kelvin and Joule. The two units covert as
1 Kelvin = 1.380649 × 10^−23 Joule
This conversion factor is called the Boltzmann constant, k.
It is analogous to
1 inch = 2.54 cm
What does the Boltzmann constant mean?
1/ Temperature has many units, among which are Kelvin and Joule. The two units covert as
1 Kelvin = 1.380649 × 10^−23 Joule
This conversion factor is called the Boltzmann constant, k.
It is analogous to
1 inch = 2.54 cm
2/ It looks silly to name an unsightly conversion factor after three great names: Joule, Kelvin, and Boltzmann.
All units are silly, but they are necessary evils.
Let's learn about the two units of temperature, Kelvin and Joule.
All units are silly, but they are necessary evils.
Let's learn about the two units of temperature, Kelvin and Joule.
3/ Entropy of an isolated system is defined as the logarithm of the number of quantum states of the isolated system:
S = log Ω
For a thermal system, which transfers energy with surroundings, entropy is a function of energy, S(U).
Temperature T is defined by
1/T = dS(U)/dU
S = log Ω
For a thermal system, which transfers energy with surroundings, entropy is a function of energy, S(U).
Temperature T is defined by
1/T = dS(U)/dU
4/ Inspect the two definitions:
S = log Ω
1/T = dS(U)/dU
We see that
-entropy is dimensionless
-temperature has the same dimension as energy, Joule.
S = log Ω
1/T = dS(U)/dU
We see that
-entropy is dimensionless
-temperature has the same dimension as energy, Joule.
5/ But Joule is a giant unit of temperature. Humans like to have a much smaller unit, Kelvin.
Kelvin had a convoluted historical definition. Since 2019, Kelvin has been defined by
1 Kelvin = 1.380649 × 10^−23 Joule
The value is set by a committee!
en.wikipedia.org/wiki/2019_rede…
Kelvin had a convoluted historical definition. Since 2019, Kelvin has been defined by
1 Kelvin = 1.380649 × 10^−23 Joule
The value is set by a committee!
en.wikipedia.org/wiki/2019_rede…
6/ Nature is indifferent to how humans define a unit for temperature.
To state any fundamental relation of nature, if temperature T is in the unit of Kelvin, the product kT must appear together.
To state any fundamental relation of nature, if temperature T is in the unit of Kelvin, the product kT must appear together.
7/ When temperature is reported in Kelvin, to maintain the equation 1/T = dS(U)/dU, the entropy need be in the fake unit of k:
S = k log Ω
If we report entropy S in this fake unit, only the ratio S/k can enter any fundamental result.
Nature is indifferent to human’s units
S = k log Ω
If we report entropy S in this fake unit, only the ratio S/k can enter any fundamental result.
Nature is indifferent to human’s units
8/ Thus, we clutter entropy with a fake unit Joule/Kelvin.
The concept of entropy is about the number of quantum states, which has nothing to do with either energy or temperature.
It takes time to explain k in
S = k log Ω
The fake idea has gone far.
The concept of entropy is about the number of quantum states, which has nothing to do with either energy or temperature.
It takes time to explain k in
S = k log Ω
The fake idea has gone far.
• • •
Missing some Tweet in this thread? You can try to
force a refresh