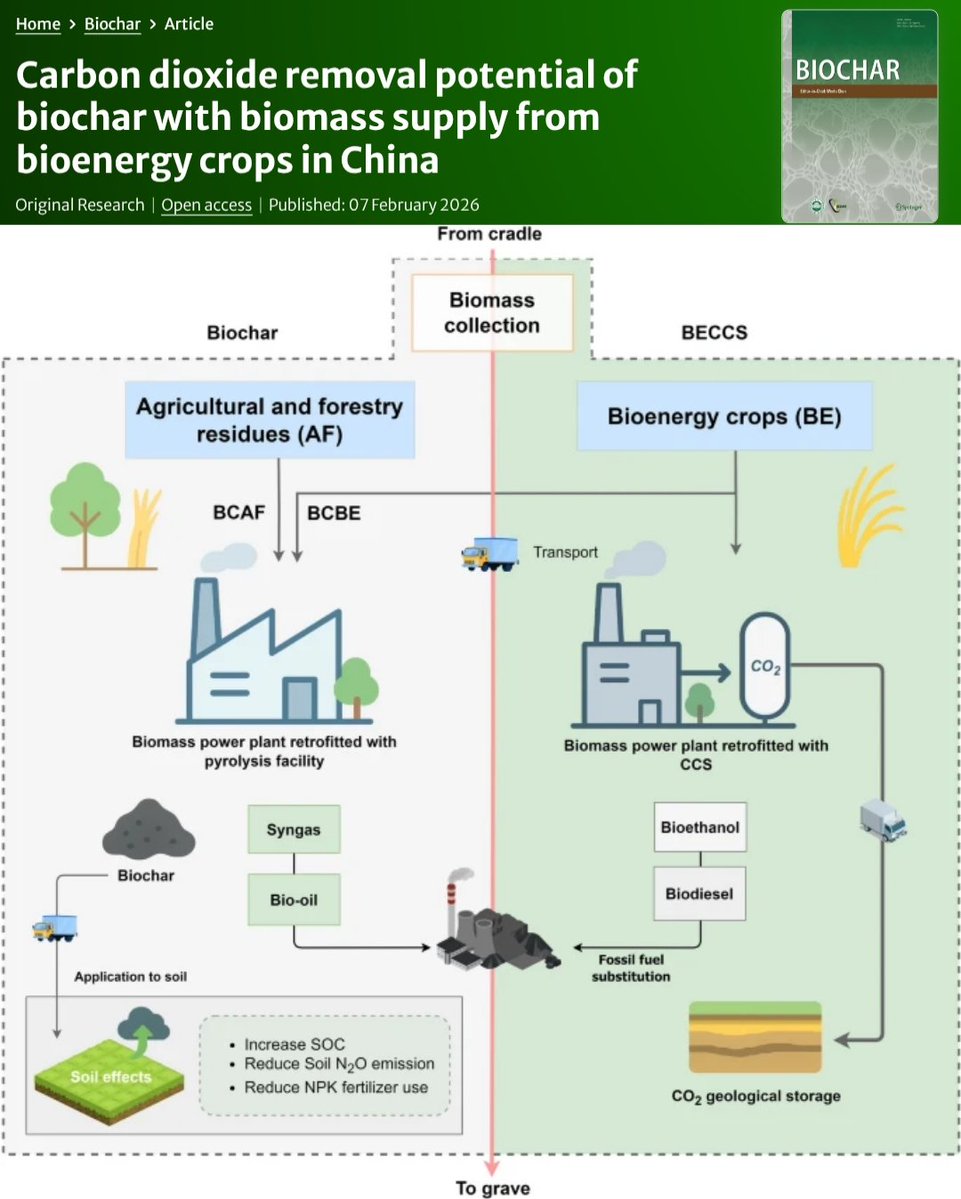
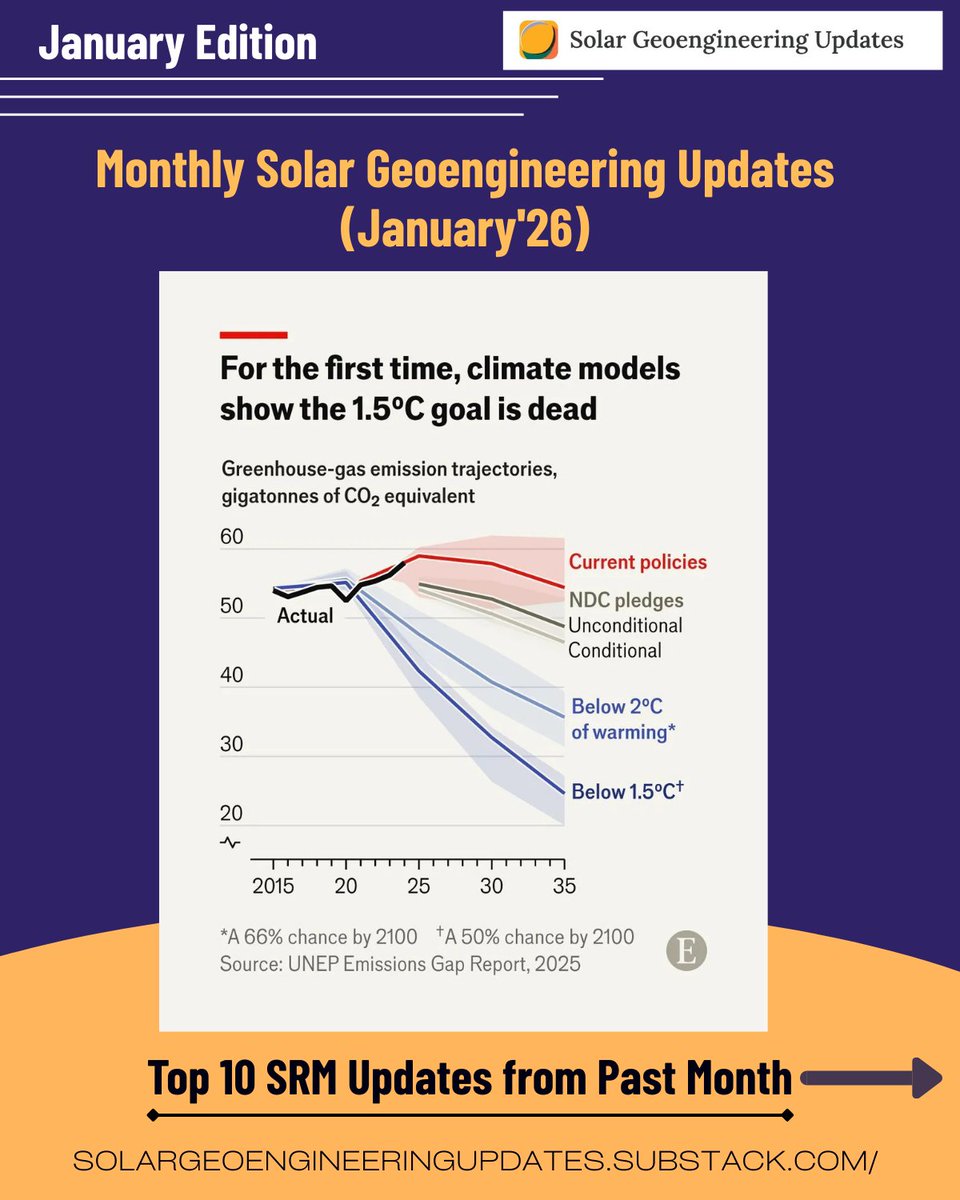
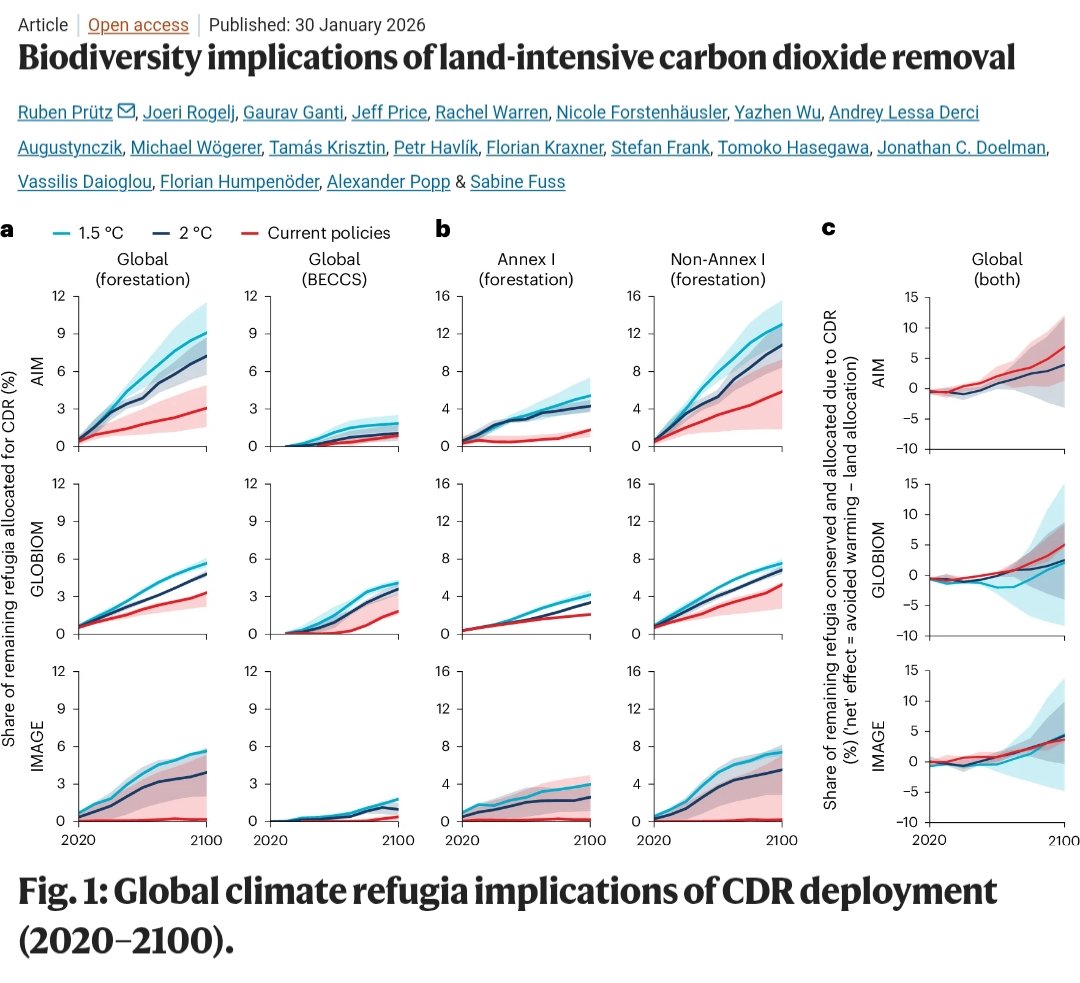
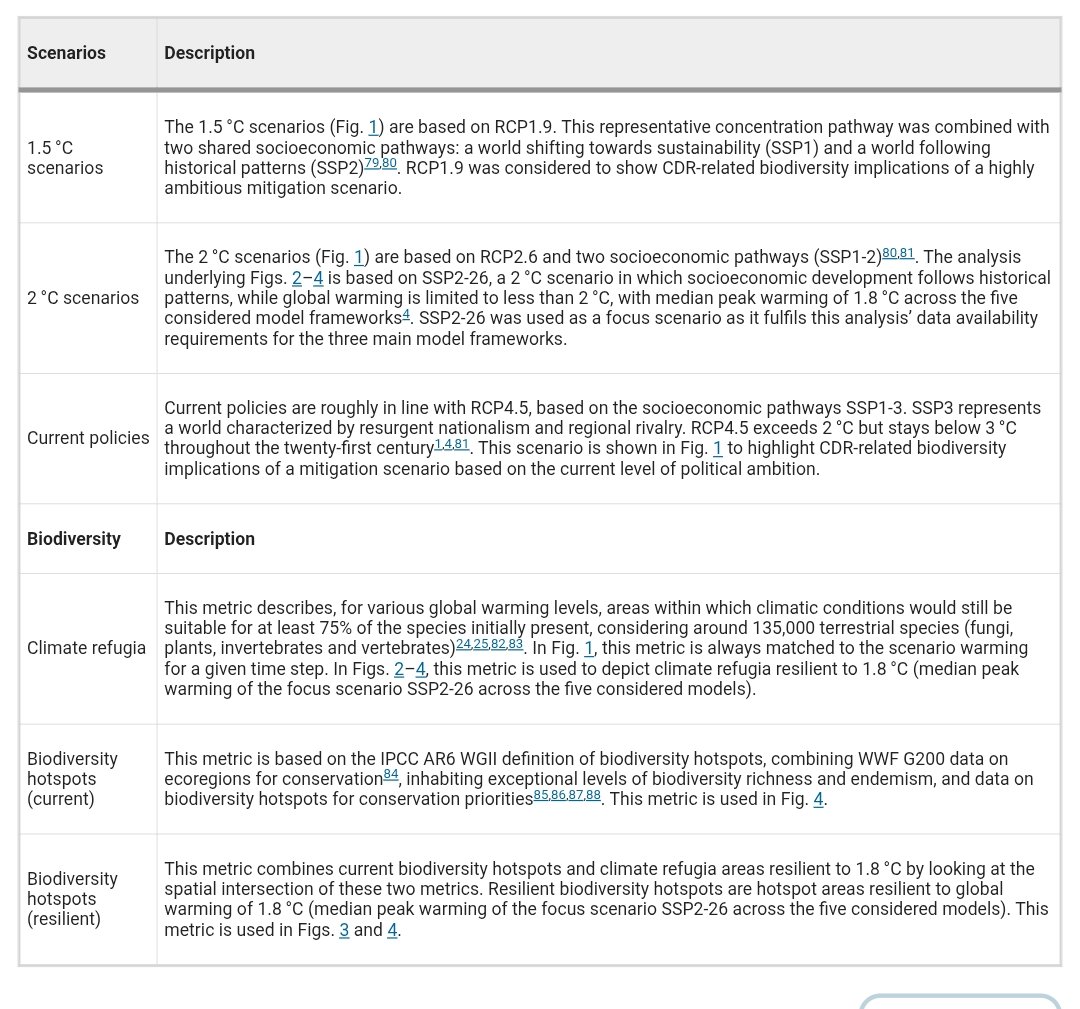
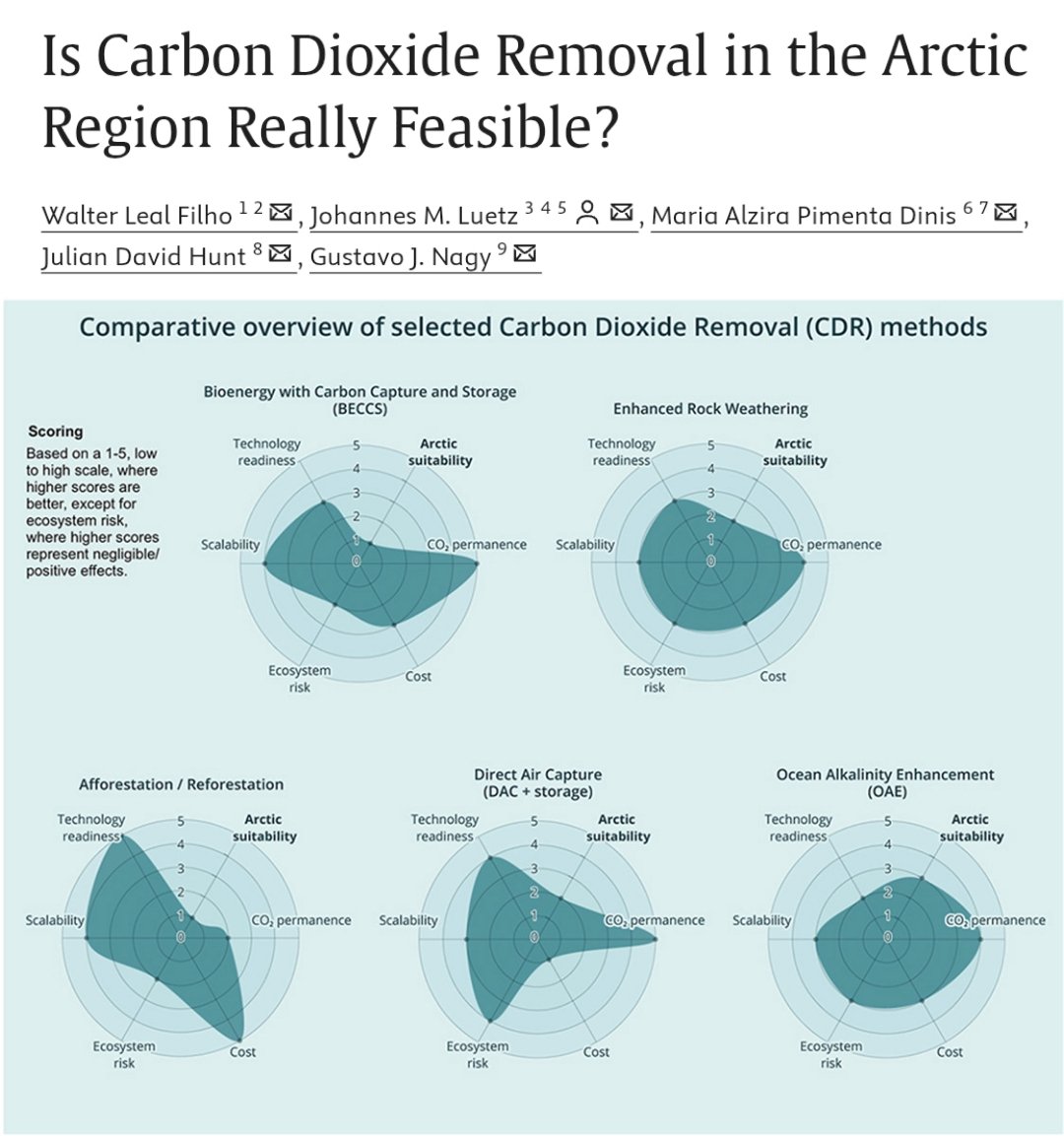
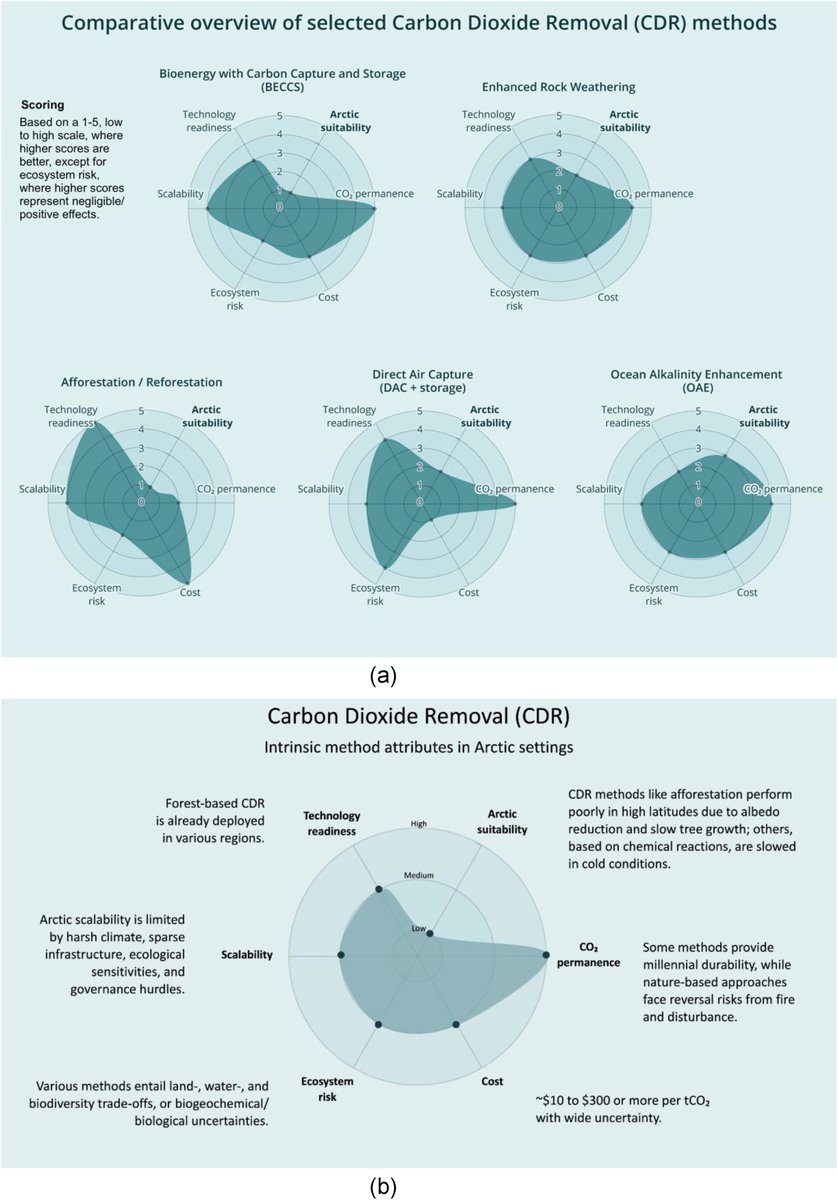
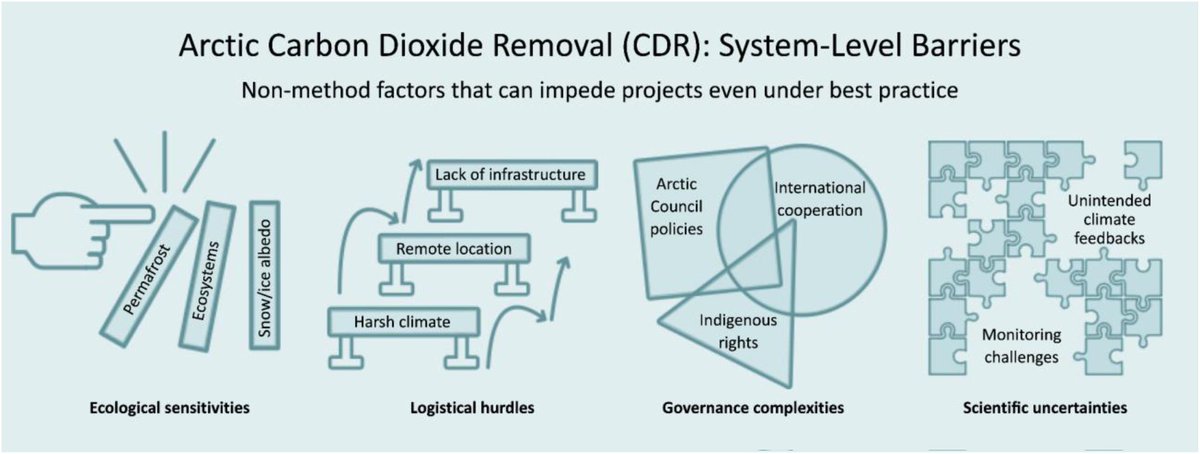
🚨PAPER🚨
#DAC of CO2 from the atm. is being explored as a tech. that can contribute to the goal of reaching #NetZero CO2 emissions.
A new study led by @DonglongFu1 showed that the use of zeolitic materials are feasible for DAC when it is integrated with H2O harvesting.
🧵
1/7
#DAC of CO2 from the atm. is being explored as a tech. that can contribute to the goal of reaching #NetZero CO2 emissions.
A new study led by @DonglongFu1 showed that the use of zeolitic materials are feasible for DAC when it is integrated with H2O harvesting.
🧵
1/7

"A combination of a commercially available desiccant, AQSOA-Z02A, and a mordenite-type zeolite (MOR) enables
continuous operation of a designed #DAC system comprised of two
parallel units with a regeneration temperature of 100°C," research finds.
2/7
continuous operation of a designed #DAC system comprised of two
parallel units with a regeneration temperature of 100°C," research finds.
2/7

Furthermore, "the system
using pure #zeolite alone requires regeneration at temperatures between 200°C and 300°C."
3/7

using pure #zeolite alone requires regeneration at temperatures between 200°C and 300°C."
3/7


"Techno-economic analyses of 12 process
scenarios reveal that the energy requirement of the best scenario
investigated is 71 GJ/tCO2, while the conventional pure zeolitebased system requires 200 GJ/tCO2."
#DAC
4/7
scenarios reveal that the energy requirement of the best scenario
investigated is 71 GJ/tCO2, while the conventional pure zeolitebased system requires 200 GJ/tCO2."
#DAC
4/7

Research declared that, "the optimized system gives a
cost between $246 and $568 per ton CO2 #captured, depending on
the energy costs, while system operates at sub-0°C temperatures or with
integration of water harvesting, respectively."
#DAC
5/7

cost between $246 and $568 per ton CO2 #captured, depending on
the energy costs, while system operates at sub-0°C temperatures or with
integration of water harvesting, respectively."
#DAC
5/7


📖 Read open-access study performed by @DonglongFu1 & Mark Devis funded by @Caltech entitled: "Toward the feasible direct air capture of
carbon dioxide with molecular sieves by water
management," here ⬇️
cell.com/cell-reports-p…
#DirectAirCapture
#CarbonDioxideRemoval
6/7
carbon dioxide with molecular sieves by water
management," here ⬇️
cell.com/cell-reports-p…
#DirectAirCapture
#CarbonDioxideRemoval
6/7
• • •
Missing some Tweet in this thread? You can try to
force a refresh