1/n Ahead of the much anticipated FDA Adcom on #lecanemab we outline key questions that physicians, payors & regulators must ask in the assessment of anti-amyloid antibodies. tinyurl.com/yrtnb2et @ProfRobHoward @kathy_y_liu @nvillain_alz @VincentPlanche @ayton_scott @SFAckley
2/n Many of these questions can be answered with existing RCT data but have not been reported by trial sponsors in peer-reviewed journals. Others may require RCTs with specific design features to test whether these are disease modifying treatments. Both incomplete & selective 

3/n reporting of RCT results as well as statistically inappropriate interpretation of existing data undermine the validity of these carefully run & challenging experiments in human patients in trial centers across the world. We specify three key questions in assessing these Rx
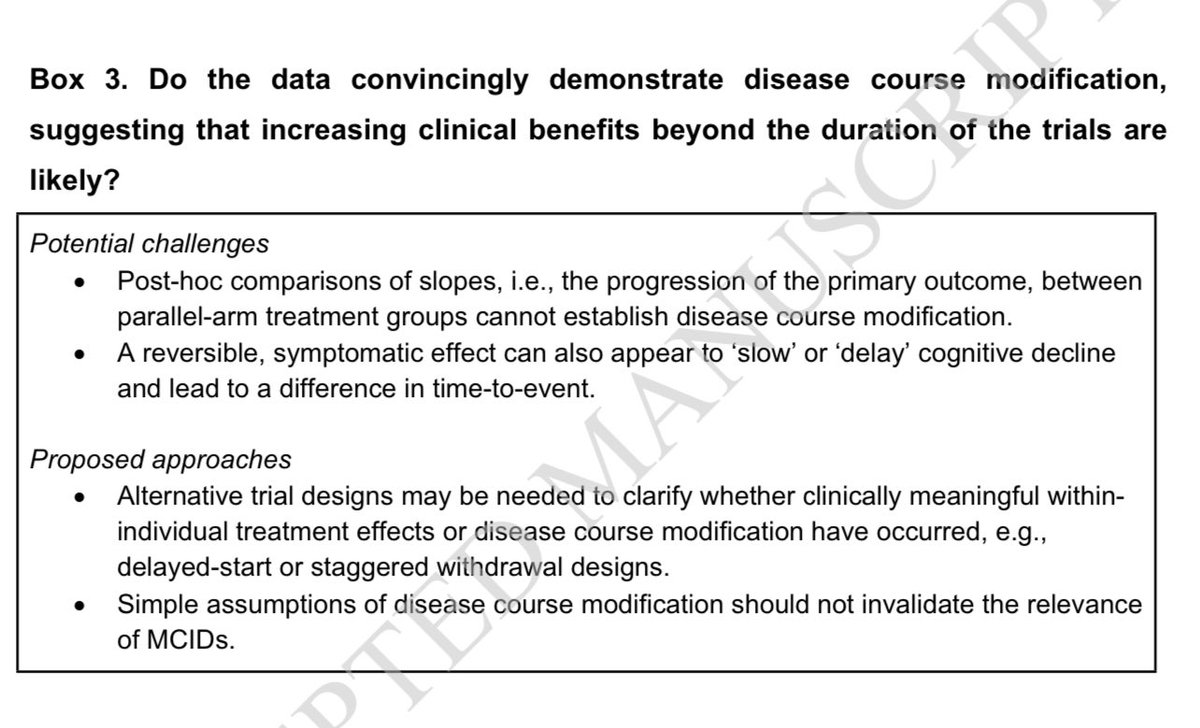
6/n Do the data convincingly demonstrate disease course modification, suggesting that increasing clinical benefits beyond the duration of the trials are likely? 
7/n The advent of a new class of licensed drugs for the treatment of Alzheimer's disease, particularly with the promise of disease course modification, represents an exciting and long-anticipated milestone for patients, caregivers, and their doctors. However, with the limited
8/n data that has been made available from clinical trials, questions over biases due to unblinding and differential drop out, as well as concerns about safety and clinical and cost-effectiveness remain. These must be addressed by regulators and payors when making approval
9/n decisions and by clinicians & patients when treatments are licensed. Hope & desperation for a treatment are not good reasons to drive such impactful decisions. We propose that attention to these three pertinent questions can guide an informed & evidence-based consideration
10/n of this important class of drugs.
A special thank you to Ms. Batool Rizvi @neurobatool for the graphical abstract in this publication.
A special thank you to Ms. Batool Rizvi @neurobatool for the graphical abstract in this publication.

• • •
Missing some Tweet in this thread? You can try to
force a refresh

 Read on Twitter
Read on Twitter











