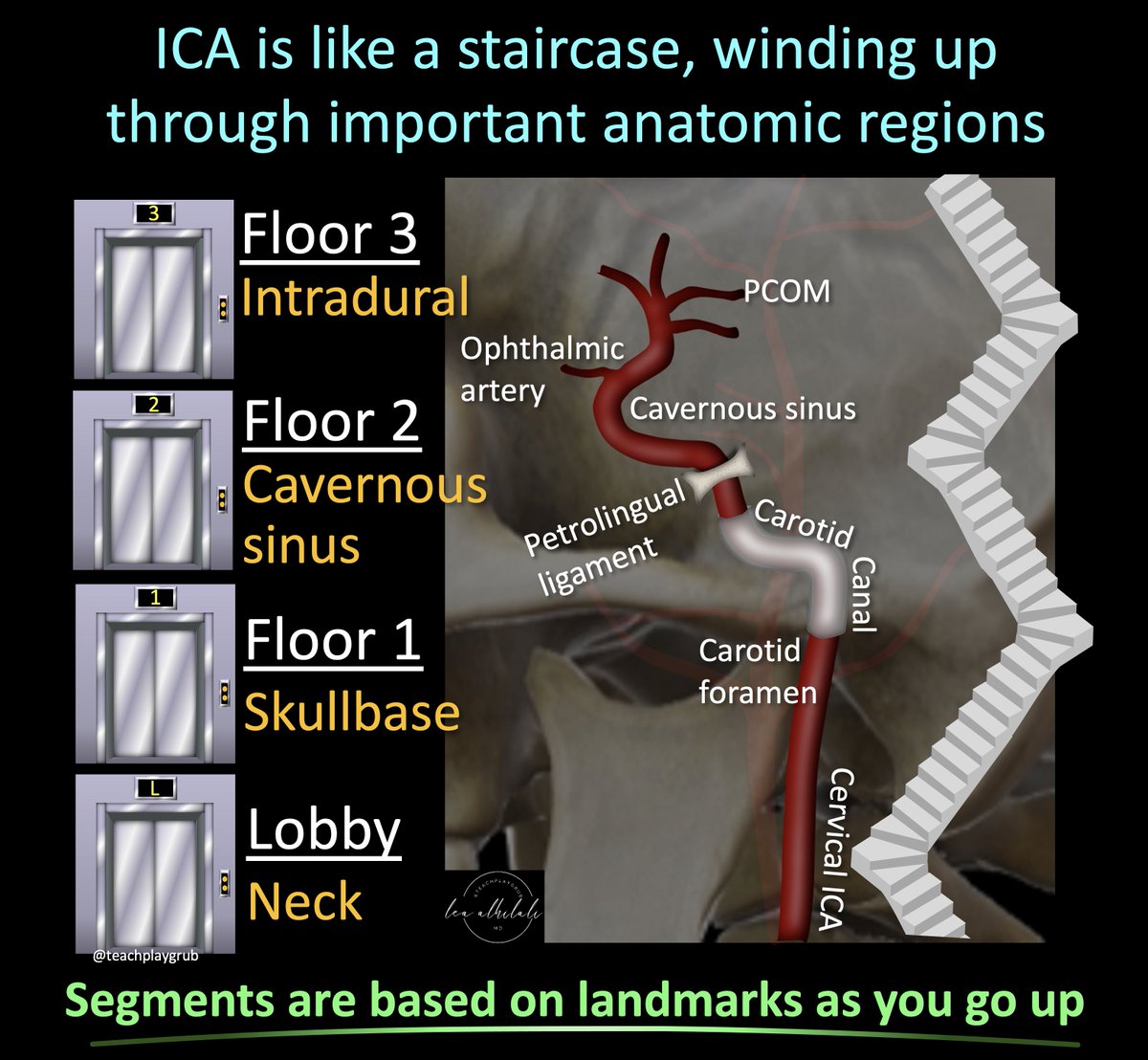
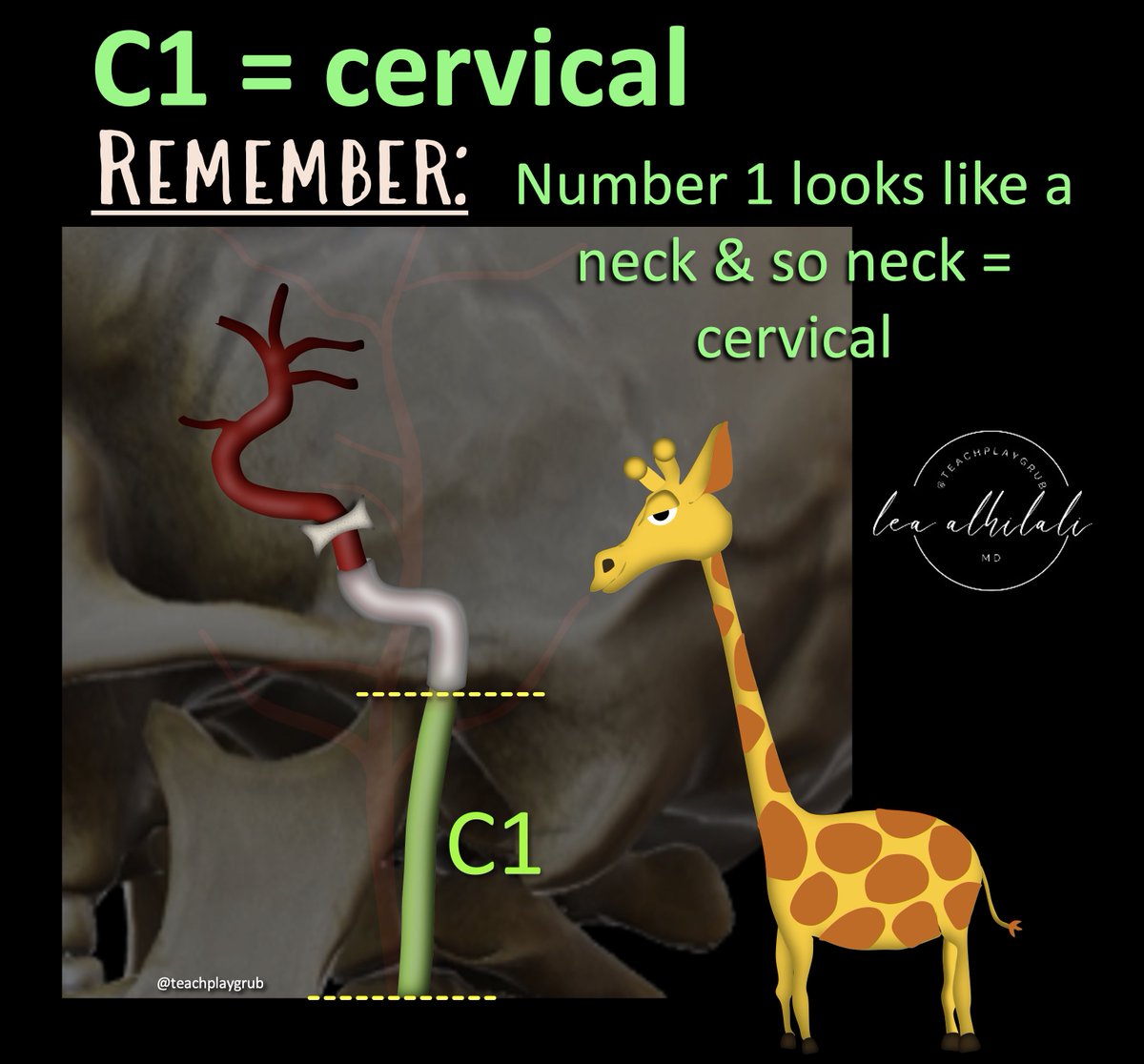
1/The 90s called & wants its carotid imaging back!
It’s been 30 years--why are you still just quoting NASCET?
A #tweetorial about carotid plaque imaging in collaboration w/ @SVINJournal!
Featuring this 🆓#openaccess article: ahajournals.org/doi/10.1161/SV…
It’s been 30 years--why are you still just quoting NASCET?
A #tweetorial about carotid plaque imaging in collaboration w/ @SVINJournal!
Featuring this 🆓#openaccess article: ahajournals.org/doi/10.1161/SV…

@SVINJournal 2/Everyone knows the NASCET criteria: If the patient is symptomatic & the greatest stenosis from the plaque is >70% of the diameter of normal distal lumen, patient will likely benefit from carotid endarterectomy. But that doesn’t mean the remaining patients are just fine! 

@SVINJournal 3/Yes, carotid plaques resulting in high grade stenosis are high risk.
But assuming that stenosis is the only mechanism by which a carotid plaque is high risk is like assuming that the only way to kill someone is by strangulation.
But assuming that stenosis is the only mechanism by which a carotid plaque is high risk is like assuming that the only way to kill someone is by strangulation.

@SVINJournal 4/Carotid disease not only harms by strangulation (stenosis), but also by serving as a source of emboli.
A gun isn’t less dangerous bc it shoots from a distance—similarly, a plaque without stenosis is still dangerous if it causes emboli, even if the harm is from a distance
A gun isn’t less dangerous bc it shoots from a distance—similarly, a plaque without stenosis is still dangerous if it causes emboli, even if the harm is from a distance

@SVINJournal 5/In fact, non-stenotic carotid plaque likely plays a key role in embolic stroke of undetermined source or ESUS.
Source may be unknown bc a full work up never found an embolic source or a full work up wasn’t completed to find a source.
We will deal w/the former
Source may be unknown bc a full work up never found an embolic source or a full work up wasn’t completed to find a source.
We will deal w/the former

@SVINJournal 6/If the ESUS involved a unilateral infarct in the anterior circulation, as many as 2 in 5 of these infarcts may be the result of emboli from non-stenotic carotid plaque 

@SVINJournal 7/This is especially true in ESUS in young patients, where other cryptogenic causes such as intermittent AFIB are rarer.
In older patients, these vulnerable plaques more commonly reach a point of stenosis, and may cause harm by both emboli and restricted flow
In older patients, these vulnerable plaques more commonly reach a point of stenosis, and may cause harm by both emboli and restricted flow

@SVINJournal 8/So how can we tell which plaques are high risk for emboli stroke and which are stable? Well, we need to need to leave NASCET behind and look at the plaque itself for clues 

@SVINJournal 9/For this, we need noninvasive imaging. Catheter angiography only looks at the lumen. We need to image the plaque itself to look for features that are associated with high risk of emboli.
CT can help look at plaque size & morphology, while MR can look at plaque composition
CT can help look at plaque size & morphology, while MR can look at plaque composition

@SVINJournal 10/The way you can remember which imaging features indicate a high-risk plaque is by remembering what foods are high risk for you to eat. If it’s bad for your bod, it’s bad for your brain. 
@SVINJournal 11/First feature is plaque size, regardless of stenosis.
Only caring about a plaque if there’s stenosis is like saying you’re only fat if your pants don’t fit.
You can be fat even if you wear big sweats that fit—big plaques can be high risk even if they fit (no stenosis)
Only caring about a plaque if there’s stenosis is like saying you’re only fat if your pants don’t fit.
You can be fat even if you wear big sweats that fit—big plaques can be high risk even if they fit (no stenosis)

@SVINJournal 12/Plaques tend to remodel outward first, so they initially don’t narrow the lumen. Only later in the course do they cause stenosis.
It's just like you wear big sweat pants to hide your dad bod, until finally you get so big those don’t fit either
It's just like you wear big sweat pants to hide your dad bod, until finally you get so big those don’t fit either

@SVINJournal 13/Plaques grow outward until fibrofatty changes restrict them, then their growth tends to press inward on the lumen.
It's just like how your dad bod pot belly grows out until your belt restricts it, then further growth tends to make your pants very tight (stenosis)
It's just like how your dad bod pot belly grows out until your belt restricts it, then further growth tends to make your pants very tight (stenosis)

@SVINJournal 14/So you can remember that plaque size is a high-risk feature bc big meals are a high-risk factor for a dad bod. No matter what the meal is composed of, eating too much puts you at risk.
Same w/plaques. No matter the composition, large absolute plaque size is a risk factor
Same w/plaques. No matter the composition, large absolute plaque size is a risk factor

@SVINJournal 15/Next is intraplaque hemorrhage. You can remember that blood in the plaque is a high risk feature bc eating rare, bloody steaks puts you at high risk for the dad bod. You don’t want to eat this = high risk feature 

@SVINJournal 16/On imaging, intraplaque hemorrhage is bright on precontrast T1 images, just like how brain hematomas are bright on precontrast T1.
MR angiograms have some T1 weighting, so plaque hemorrhage is a feature you can see on routine MRA—it will be bright outside the lumen!
MR angiograms have some T1 weighting, so plaque hemorrhage is a feature you can see on routine MRA—it will be bright outside the lumen!

@SVINJournal 17/Next is plaque irregularity.
Just like how you wouldn't want to eat something that someone had pressed their finger into—if the plaque looks like it has lots of fingerprint indentations, it's high risk.
You can see this on just a routine CT angiogram.
Just like how you wouldn't want to eat something that someone had pressed their finger into—if the plaque looks like it has lots of fingerprint indentations, it's high risk.
You can see this on just a routine CT angiogram.

@SVINJournal 18/Next is plaque ulceration.
This is a step up from irregularity. Now someone hasn’t just stuck their finger in your food—they have taken a bite out of it! You certainly don’t want to eat that!
You can see these bites out of the plaque on routine CT angiograms.
This is a step up from irregularity. Now someone hasn’t just stuck their finger in your food—they have taken a bite out of it! You certainly don’t want to eat that!
You can see these bites out of the plaque on routine CT angiograms.

@SVINJournal 19/Next is a lipid rich necrotic core.
You can remember that lipid/fat in the plaque is a high risk features b/c eating rich, fatty foods puts you at high risk for the dad bod.
Remember, if you don’t want to eat it—you don’t want it in your carotid plaque either!
You can remember that lipid/fat in the plaque is a high risk features b/c eating rich, fatty foods puts you at high risk for the dad bod.
Remember, if you don’t want to eat it—you don’t want it in your carotid plaque either!

@SVINJournal 20/To see the lipid core, we need black blood MRI.
Black blood MR nulls the signal in the vessels so they’re black (hence the name “black blood”). This helps us to see the plaque surface & enhancement, which would otherwise be covered up by the signal from the vessel
Black blood MR nulls the signal in the vessels so they’re black (hence the name “black blood”). This helps us to see the plaque surface & enhancement, which would otherwise be covered up by the signal from the vessel

@SVINJournal 21/Black blood MR is often the same sequence used on an MR dissection protocol—you just add contrast to see regions of inflammation & vascularity
On black blood MR, the lipid rich necrotic core looks just how necrotic things look in the brain—low signal, w/non-enhancing core
On black blood MR, the lipid rich necrotic core looks just how necrotic things look in the brain—low signal, w/non-enhancing core

@SVINJournal 22/Next is plaque neovascularity.
Just like how you wouldn’t want to eat food where new moldy things were growing into it—you don’t want a plaque that has new blood vessels growing into it.
You can see neovascularity as adventitial enhancement on black blood MRI.
Just like how you wouldn’t want to eat food where new moldy things were growing into it—you don’t want a plaque that has new blood vessels growing into it.
You can see neovascularity as adventitial enhancement on black blood MRI.

@SVINJournal 23/Finally is loss of the normal fibrous cap.
Think of the fibrous cap covering like ziplock bag, keeping everything clean & fresh. If there’s a tear in the bag, well, that food is kind of sketchy now. Similarly, you don’t want a plaque w/a tear in its protective covering
Think of the fibrous cap covering like ziplock bag, keeping everything clean & fresh. If there’s a tear in the bag, well, that food is kind of sketchy now. Similarly, you don’t want a plaque w/a tear in its protective covering

@SVINJournal 24/Normally, the fibrous cap enhances on black blood MRI bc it has vascular fibrous tissue.
Thinning or loss of this normal enhancing margin between the lumen and the plaque indicates a high risk plaque.
Thinning or loss of this normal enhancing margin between the lumen and the plaque indicates a high risk plaque.

@SVINJournal 25/So go beyond NASCET!
Hopefully, now you’ll never feel vulnerable when it comes to carotid plaque imaging!
Be sure to check out the excellent review by @JimSiegler on non-stenotic carotid plaques featured in @SVINJournal!
ahajournals.org/doi/10.1161/SV…
Hopefully, now you’ll never feel vulnerable when it comes to carotid plaque imaging!
Be sure to check out the excellent review by @JimSiegler on non-stenotic carotid plaques featured in @SVINJournal!
ahajournals.org/doi/10.1161/SV…

• • •
Missing some Tweet in this thread? You can try to
force a refresh