The serum free light chain FLC assay. Tutorial. #MedTwitter #MyelomaVR
Frequently ordered and frequently abnormal. This thread will reduce unnecessary headaches and referrals.
1/ Don’t order it unless you are suspecting myeloma, amyloid or related disorder
Frequently ordered and frequently abnormal. This thread will reduce unnecessary headaches and referrals.
1/ Don’t order it unless you are suspecting myeloma, amyloid or related disorder
2/ The assay itself consists of 2 separate assays: a free kappa assay and a free lambda assay.
The lab also CALCULATES and reports a free kappa/lambda light chain ratio using the absolute levels.
The lab also CALCULATES and reports a free kappa/lambda light chain ratio using the absolute levels.
4) The serum FLC assay is a brilliantly designed. Binds to epitopes hidden in intact immunoglobulin. So it doesn’t pick up the large quantities of kappa/lambda light chains that are present on intact immunoglobulins like IgG/IgA/IgM.
It only detects unbound light chains.
It only detects unbound light chains.

5) The serum FLC assay is more sensitive than serum immunofixation is detecting free (unbound) circulating monoclonal light chains. But it is NOT more sensitive and in fact cannot pick up intact monoclonal proteins. So for eg., it picks up free kappa but not IgG kappa.
6) We all make a slight excess of free kappa and free lambda light chains. So we all have small measurable levels. And we have a normal range for the kappa/ lambda ratio.
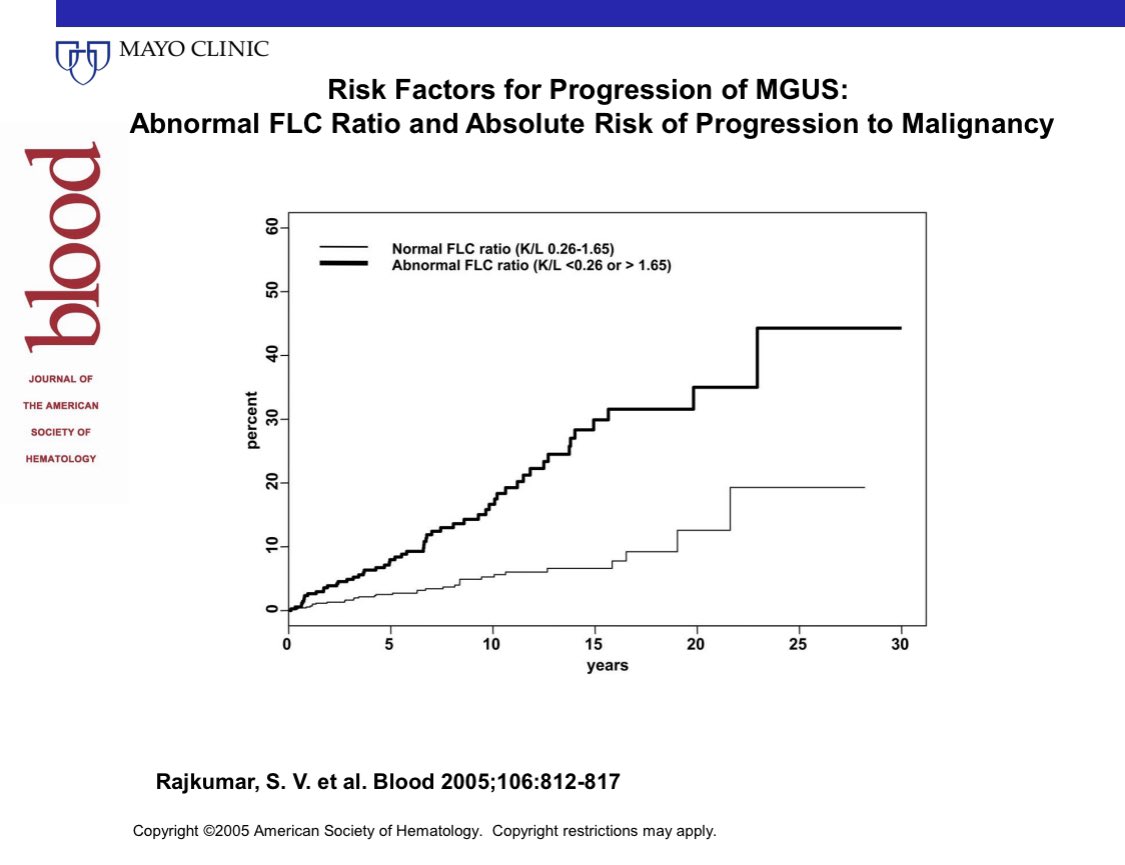
For the Binding Site free light chain assay the normal kappa/lambda ratio is reported as 0.26-1.65
For the Binding Site free light chain assay the normal kappa/lambda ratio is reported as 0.26-1.65
7) The normal FLC levels and FLC ratio and range are not set in stone. They were established using levels in young normal volunteers. But we know now that in renal failure and with increasing age the true normal range is wider.
So don’t lose sleep over slight abnormalities.
So don’t lose sleep over slight abnormalities.
8) When the kappa/lambda FLC ratio is more than the upper limit, especially >4, it means there is likely a monoclonal kappa plasma cell disorder.
When the kappa/lambda ratio is less than the lower limit, especially <0.125, it means a monoclonal lambda plasma cell disorder.
When the kappa/lambda ratio is less than the lower limit, especially <0.125, it means a monoclonal lambda plasma cell disorder.
9) I’m not good at math. So rather than the kappa/lambda ratio the lab reports, I do involved/uninvolved ratio which provides a nice whole number.
So take the higher level and divide by lower level. This involved/uninvolved ratio is what we use for risk stratification.
So take the higher level and divide by lower level. This involved/uninvolved ratio is what we use for risk stratification.
10) So we can use a involved/uninvolved ratio >4 to say whether there is a monoclonal plasma cell disorder or not.
Or a ratio >20 to say whether smoldering myeloma is high or low risk.
Or a ratio of >100 to say whether someone has myeloma defining event or not.
Or a ratio >20 to say whether smoldering myeloma is high or low risk.
Or a ratio of >100 to say whether someone has myeloma defining event or not.
11) FLC Assay USE #1
To follow patients with myeloma and other plasma cell disorders
We follow the level & ratio to assess response to therapy & to detect progression. We have specific cut offs for how much reduction qualifies for response & how much qualifies for progression
To follow patients with myeloma and other plasma cell disorders
We follow the level & ratio to assess response to therapy & to detect progression. We have specific cut offs for how much reduction qualifies for response & how much qualifies for progression
12) FLC Assay USE #2
To diagnose whether patients have a monoclonal plasma cell disorder or not.
It complements serum protein electrophoresis and immunofixation. For myeloma, without FLC assay, sensitivity is 93%. If you also do the FLC assay your sensitivity increases to 98%
To diagnose whether patients have a monoclonal plasma cell disorder or not.
It complements serum protein electrophoresis and immunofixation. For myeloma, without FLC assay, sensitivity is 93%. If you also do the FLC assay your sensitivity increases to 98%
13) FLC assay USE#3
To risk stratify MGUS
1/3 of patients with MGUS have an abnormal FLC ratio. The risk of progression increases to ~3% per year rather than the <1% per year for MGUS patients who have a normal FLC ratio.
To risk stratify MGUS
1/3 of patients with MGUS have an abnormal FLC ratio. The risk of progression increases to ~3% per year rather than the <1% per year for MGUS patients who have a normal FLC ratio.
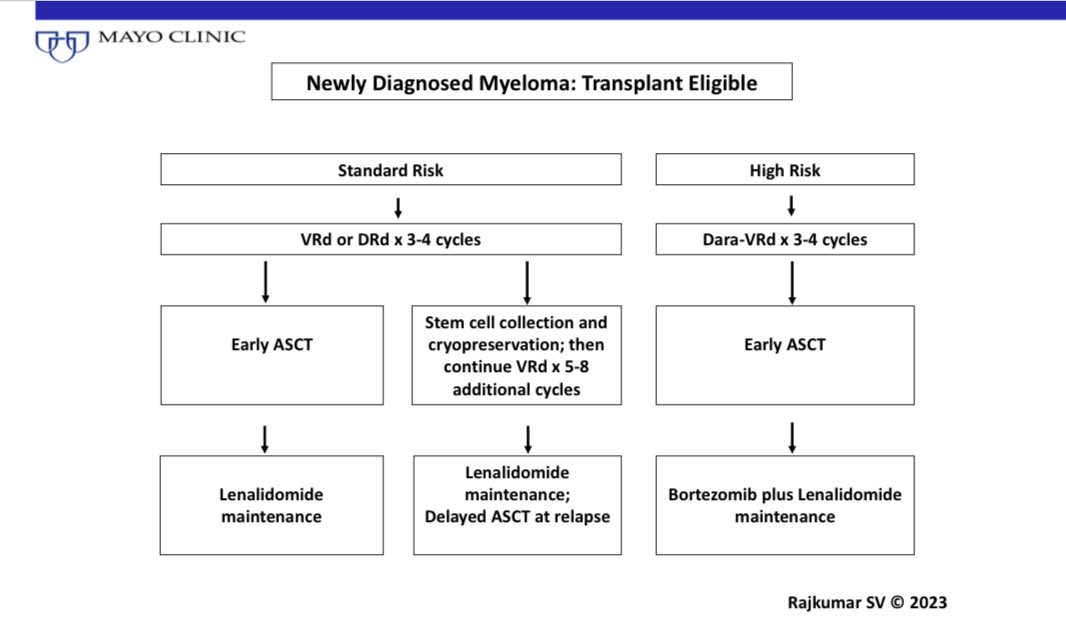
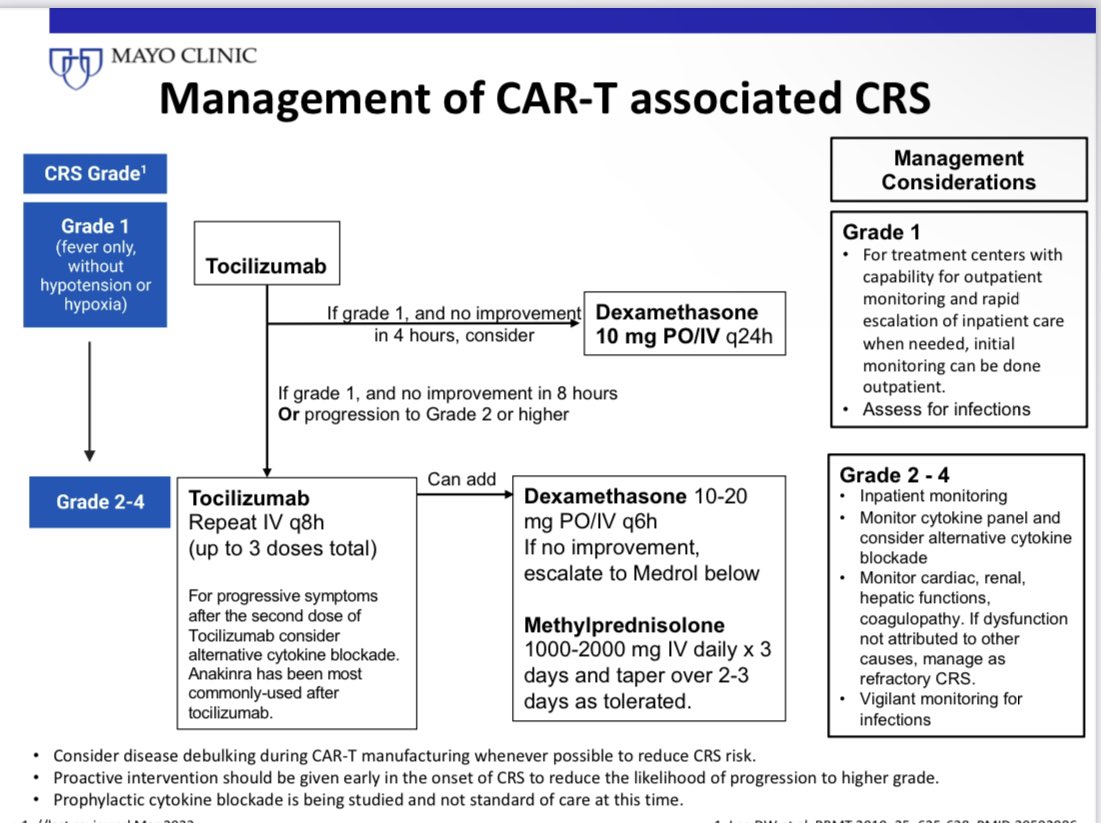
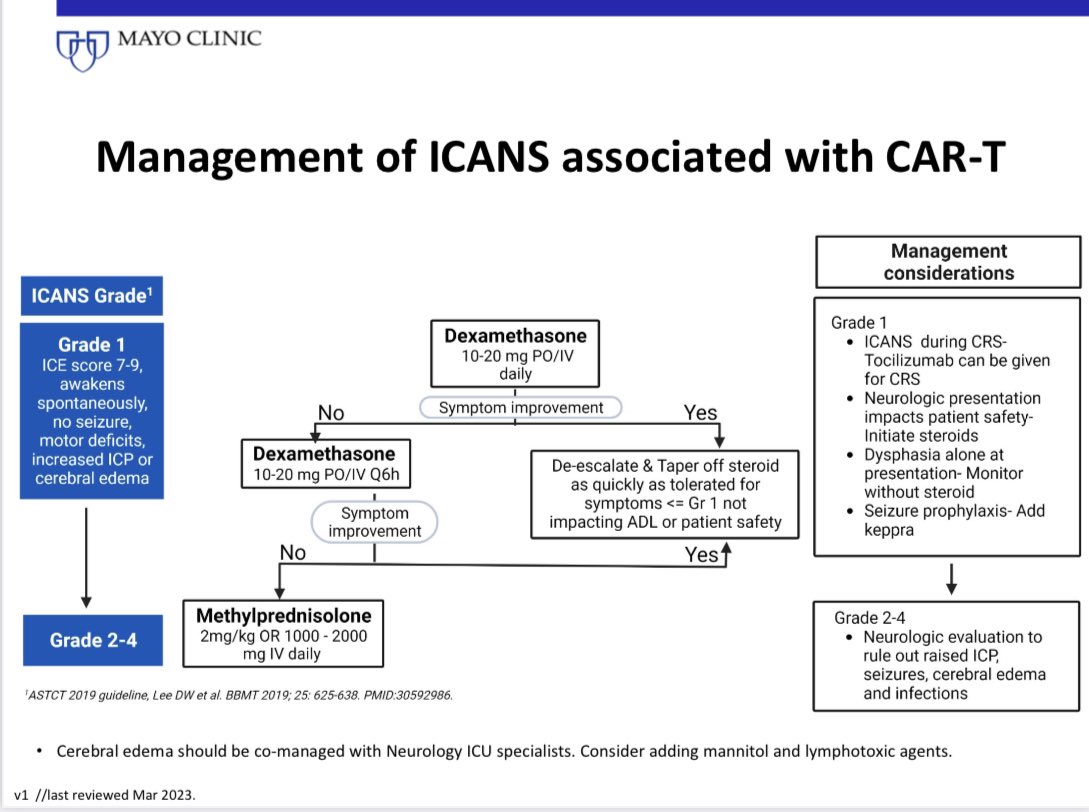
14) FLC assay USE #4
Risk stratify SMM. 1/3 of patients with smoldering myeloma have an involved/uninvolved FLC ratio >20.
This cut off is used to risk stratify SMM. The risk of progression in SMM increases with increasing ratio, and we use a cut off off of >20.
Risk stratify SMM. 1/3 of patients with smoldering myeloma have an involved/uninvolved FLC ratio >20.
This cut off is used to risk stratify SMM. The risk of progression in SMM increases with increasing ratio, and we use a cut off off of >20.
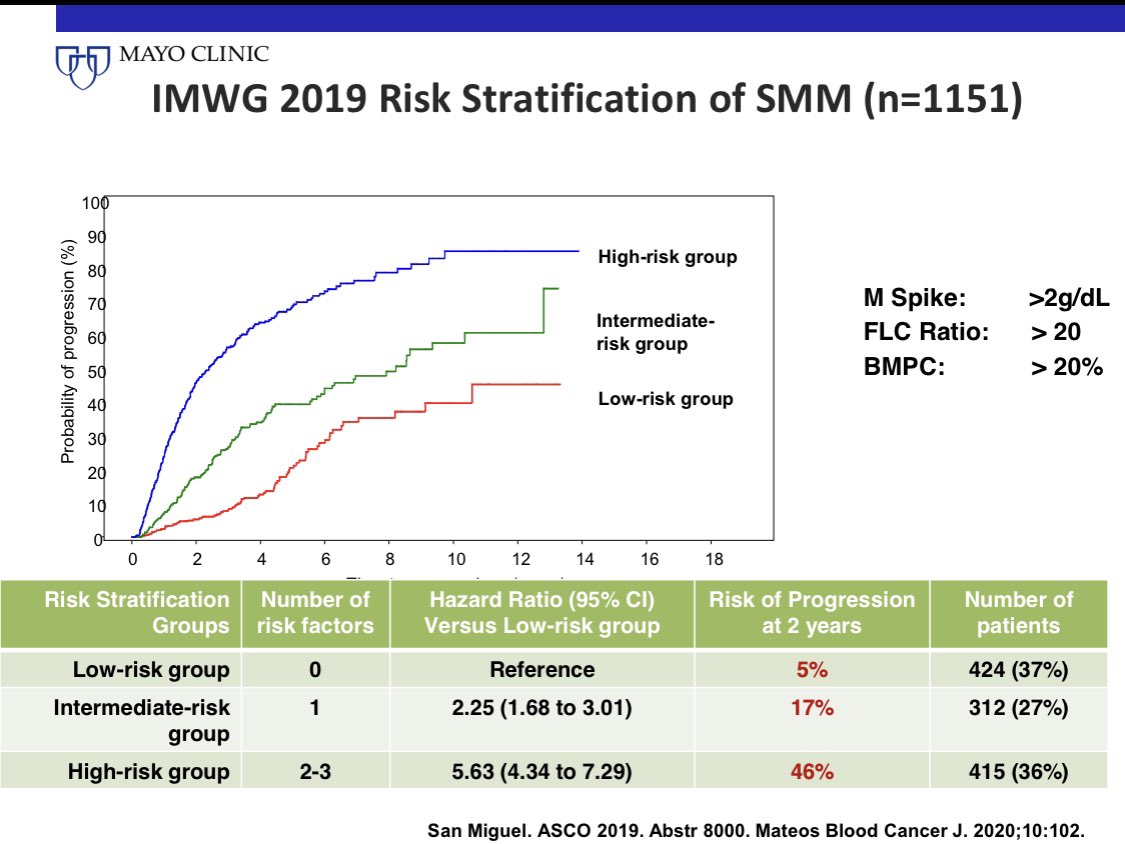
15) FLC assay USE #5
As a myeloma defining event. To diagnose myeloma.
An involved/uninvolved ratio of >100 coupled with an absolute involved level of >100 mg/L, and urine M protein >200 mg/24 H is a myeloma defining event.
As a myeloma defining event. To diagnose myeloma.
An involved/uninvolved ratio of >100 coupled with an absolute involved level of >100 mg/L, and urine M protein >200 mg/24 H is a myeloma defining event.
16) FLC Assay Use #6
To diagnose light chain MGUS. Here the serum & urine immunofixation do not show any heavy chain expression. ie. there is no IgG or IgA or IgM M protein. The clonal plasma cells are only making light chains.
Unless ratio is >8, risk of progression is low.
To diagnose light chain MGUS. Here the serum & urine immunofixation do not show any heavy chain expression. ie. there is no IgG or IgA or IgM M protein. The clonal plasma cells are only making light chains.
Unless ratio is >8, risk of progression is low.
17) FLC assay Use #7
Prognosis of solitary plasmacytoma after radiation.
If the FLC ratio doesn’t normalize by one year after radiation, the risk of progression or relapse is higher.
Prognosis of solitary plasmacytoma after radiation.
If the FLC ratio doesn’t normalize by one year after radiation, the risk of progression or relapse is higher.
18) FLC assay Caveat #1
Some labs report the absolute level in mg/L. Some in mg/dl
Please check units when following. Otherwise you may think there is a massive change when all that’s happened is the lab changed.
Some labs report the absolute level in mg/L. Some in mg/dl
Please check units when following. Otherwise you may think there is a massive change when all that’s happened is the lab changed.
19) FLC assay Caveat #2
The ratio can be affected greatly and swing wildly if the uninvolved normal light chain (lambda in a patient with kappa myeloma for example) is very low. So follow both the actual level and the ratio. Not just the ratio.
The ratio can be affected greatly and swing wildly if the uninvolved normal light chain (lambda in a patient with kappa myeloma for example) is very low. So follow both the actual level and the ratio. Not just the ratio.
20) FLC assay caveat #3
You need a change of >10 mg/dl or 100 mg/L to be sure that it’s a real change and not a lab variation in most settings. Except in amyloid small changes are not very reliable.
You need a change of >10 mg/dl or 100 mg/L to be sure that it’s a real change and not a lab variation in most settings. Except in amyloid small changes are not very reliable.
21) FLC assay caveat #4
Some people don’t excrete the light chain due to dimerization. So a high level may be related to decreased excretion and not increased production. To verify check urine M spike. To consider as myeloma defining event, you need urine M spike >200 mg/24h
Some people don’t excrete the light chain due to dimerization. So a high level may be related to decreased excretion and not increased production. To verify check urine M spike. To consider as myeloma defining event, you need urine M spike >200 mg/24h
22) FLC assay caveat #5
The levels and even the ratio may be perturbed by renal failure. Usually both light chains are higher. nature.com/articles/s4140…
The levels and even the ratio may be perturbed by renal failure. Usually both light chains are higher. nature.com/articles/s4140…
23) FLC assay caveat #6
Like the sed rate, FLC levels increase in any inflammatory process. Both kappa and lambda levels go up. Ratio is normal or only mildly perturbed. This is polyclonal process. Not monoclonal gammopathy.
Like the sed rate, FLC levels increase in any inflammatory process. Both kappa and lambda levels go up. Ratio is normal or only mildly perturbed. This is polyclonal process. Not monoclonal gammopathy.
24) Algorithm for using FLC assay in managing MGUS. When to refer. How to follow. When can follow up be deferred. When can Bone marrow biopsy and bone imaging be deferred. #MedTwitter #MGUSVR 
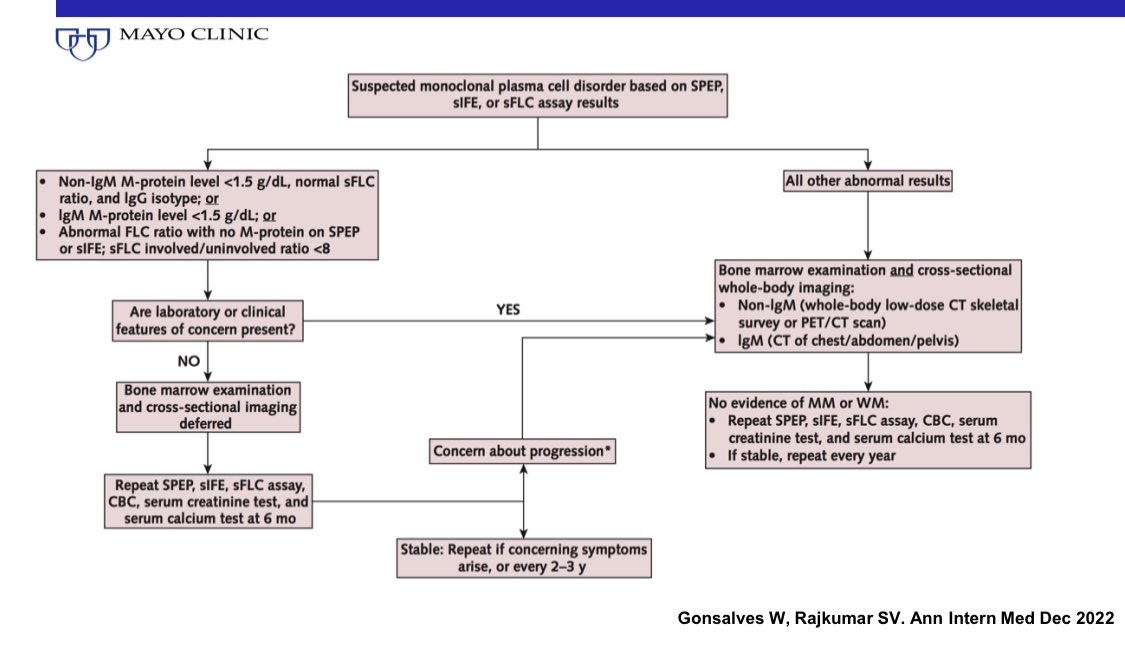
@fabianeac We also follow FLC levels once a year in MGUS and once every 3-4 months in SMM in addition to SPEP.
The algorithm provides guidance for following patients with very low risk of progression.
The algorithm provides guidance for following patients with very low risk of progression.
@Filippo_Bagnoli The rationale for FLC ratio >100 is to identify patients who make light chains in large enough quantities that it indicates presence of malignancy (rather than MGUS) and the fact that at high levels the light chains cause cast nephropathy.
@Filippo_Bagnoli If absolute level is not high, there is no concern for a malignancy-level high production or risk of renal failure.
Similarly if FLC level is high because FLCs are too Bugg to be filtered out by the kidney, it’s not a high production problem or a risk for renal failure.
Similarly if FLC level is high because FLCs are too Bugg to be filtered out by the kidney, it’s not a high production problem or a risk for renal failure.
• • •
Missing some Tweet in this thread? You can try to
force a refresh

 Read on Twitter
Read on Twitter