A short 🧵on a recent study by @MaggieLind2 with @MHitchingsEpi @datcummings Albert Ko et al. Data show that immunity induced by vaccines, prior infection or both (hybrid) protects against SARS-CoV-2 infection when viral exposure is low to moderate (1/)
nature.com/articles/s4146…
nature.com/articles/s4146…
Question being asked: What is the risk of becoming *infected* with SARS-CoV-2 after developing immunity following a vaccine, prior infection, or both if exposure to the virus is very high, moderate, or low? They did not study the severity of symptoms. (2/)
How? The authors used the existing database of the Connecticut Department of Correction, where infection data based on high frequency of testing for SARS-CoV-2 on ~9300 residents across 13 facilities were available. (3/)
What did they find? Prior infection, vaccination, or both provided significant protection against infection when the exposure was moderate (index case was within their cellblock) or low (no exposure was documented in cellblock co-residents) against Delta or Omicron. (4/) 

However, when the viral exposure was intense (with infected cellmate - exposure is 24/7), none of these groups had enough immunity to protect against infection with Delta or Omicron virus. (5/) 
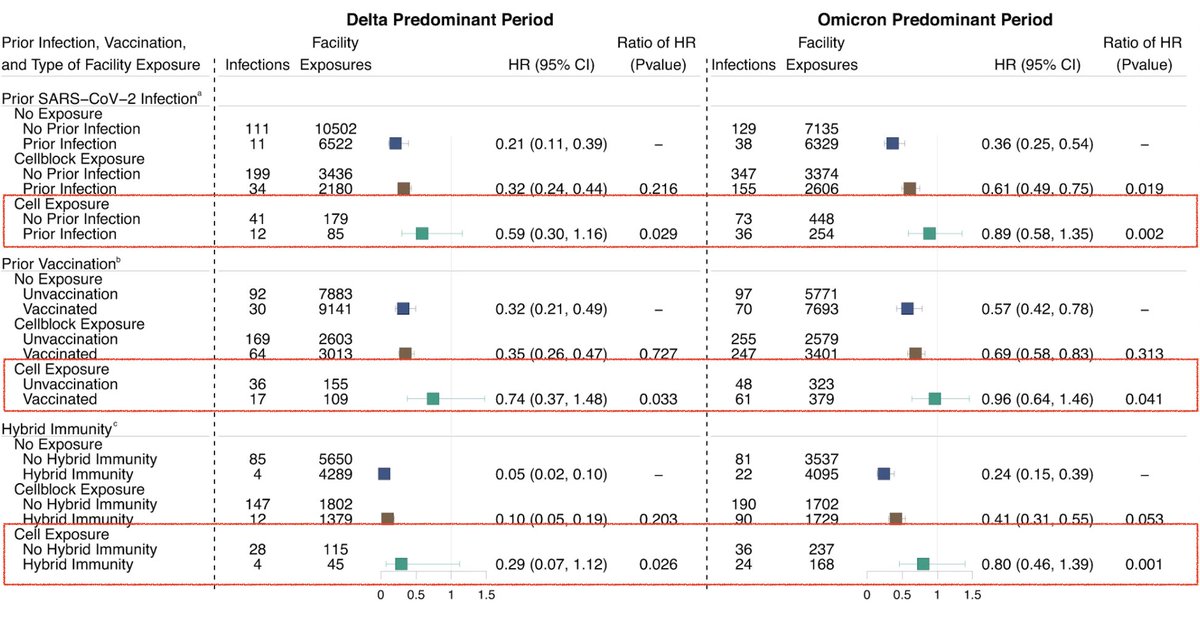
These findings suggest that protection conferred by prior infection and vaccination is dependent on the cumulative viral exposure dose. An important question for the future is to determine how much the viral load vs. duration of exposure plays a key role. (6/)
If the protection is indeed dose-dependent, coupling non-pharmaceutical interventions with vaccination would be beneficial because the non-pharmaceutical intervention (masking, ventilation, etc..) reduces viral exposure, resulting in improved levels of conferred protection. (7/)
More broadly, this study answers a fundamental question on the nature of immunity. While the field speculated that immune protection against infection is exposure dose-dependent, data in support were limited. Now this study directly demonstrated this for COVID. (8/)
Their findings suggest the “need for layered interventions to mitigate SARS-CoV-2 spread, especially within dense settings, such as congregate settings, and in settings where prolonged contact is likely, such as households with infected people.” An important message to end 🧵
• • •
Missing some Tweet in this thread? You can try to
force a refresh

 Read on Twitter
Read on Twitter