少し調べてみました。今後の調査の参考になればと思いますが、不明な点だらけですので軽い気持ちでお読み下さい。
1.論文
2.治験概要資料
3.論文補足資料
4.PMDA資料
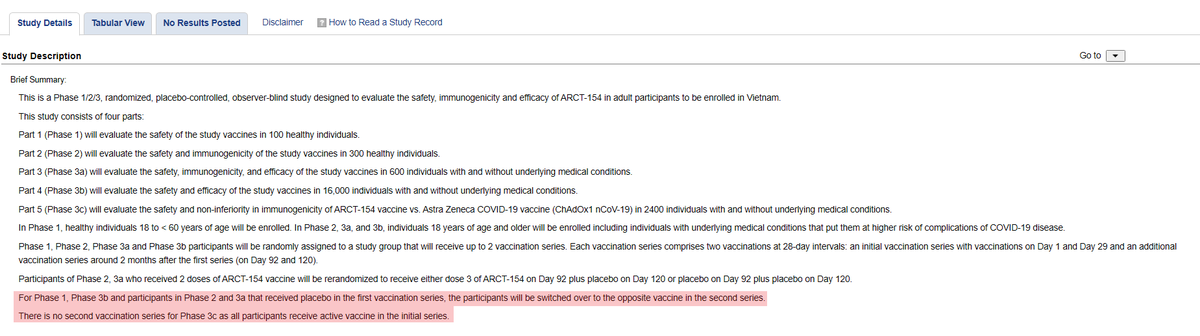
論文で死亡例が言及された「フェーズ3b」は、第1シリーズ(1日・29日の2回接種)と第2シリーズ(92日・120日の2回接種)に分かれていて、第1と第2で接種対象が入れ替わるようです。
第1シ:ワクチン2回→第2シ:プラセボ2回
第1シ:プラセボ2回→第2シ:ワクチン2回
イメージはPMDA資料と治験概要資料からnature.com/articles/s4146…
classic.clinicaltrials.gov/ct2/show/NCT05…
static-content.springer.com/esm/art%3A10.1…
pmda.go.jp/drugs/2023/P20…

1.論文
2.治験概要資料
3.論文補足資料
4.PMDA資料
論文で死亡例が言及された「フェーズ3b」は、第1シリーズ(1日・29日の2回接種)と第2シリーズ(92日・120日の2回接種)に分かれていて、第1と第2で接種対象が入れ替わるようです。
第1シ:ワクチン2回→第2シ:プラセボ2回
第1シ:プラセボ2回→第2シ:ワクチン2回
イメージはPMDA資料と治験概要資料からnature.com/articles/s4146…
classic.clinicaltrials.gov/ct2/show/NCT05…
static-content.springer.com/esm/art%3A10.1…
pmda.go.jp/drugs/2023/P20…

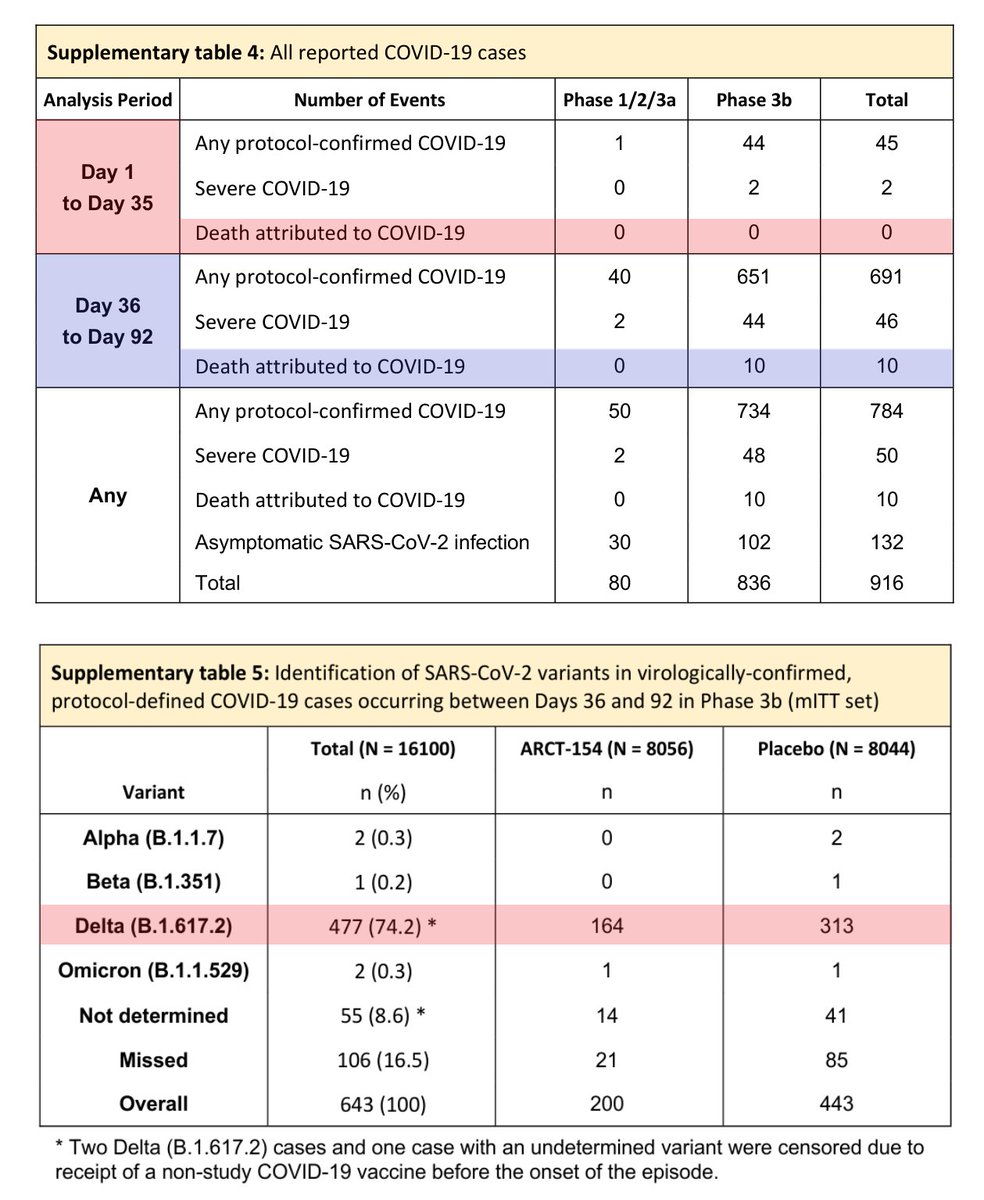
論文では、フェーズ3bで、ワクチン接種者5名、プラセボ接種者16名の計21名が死亡したと報告されています。これは、第1シリーズ後の結果で、第2シリーズの結果は含まれていないようです。
21件のうち10件がCOVID-19による感染死です。
内訳
ワクチン接種者:5人中1人が感染死、4人は別死因
プラセボ接種者:16人中9人が感染死、7人は別死因
治験(フェーズ3b)での死亡の定義は、「治験概要」に記載されているのですが、この定義通り運用されているのかは、確信を持てません。
治験概要
副次的結果の評価
9. COVID-19による死亡者の参加者数[期間:37日目から92日目]
プールされたフェーズ1/2/3a/3bフェーズ3b参加者:研究ワクチンの2回目投与の7日後を起点に、以前の感染の証拠がない参加者におけるCOVID-19による死亡が発生した参加者数。
この定義で運用される場合、第1シリーズの2回接種を1セットとして7日後から感染死のカウントがスタートすると解釈されます。除外された感染死者が存在する場合、別の死因に分類されるのでしょうか?
(1)36日までの感染死は除外?
(2)37日から92日で既感染者の感染死は除外?
(3)2回目投与から7日までの感染死は除外?
(4)1回目接種後の感染死は除外?
(5)92日以降は未カウント?classic.clinicaltrials.gov/ct2/show/NCT05…

21件のうち10件がCOVID-19による感染死です。
内訳
ワクチン接種者:5人中1人が感染死、4人は別死因
プラセボ接種者:16人中9人が感染死、7人は別死因
治験(フェーズ3b)での死亡の定義は、「治験概要」に記載されているのですが、この定義通り運用されているのかは、確信を持てません。
治験概要
副次的結果の評価
9. COVID-19による死亡者の参加者数[期間:37日目から92日目]
プールされたフェーズ1/2/3a/3bフェーズ3b参加者:研究ワクチンの2回目投与の7日後を起点に、以前の感染の証拠がない参加者におけるCOVID-19による死亡が発生した参加者数。
この定義で運用される場合、第1シリーズの2回接種を1セットとして7日後から感染死のカウントがスタートすると解釈されます。除外された感染死者が存在する場合、別の死因に分類されるのでしょうか?
(1)36日までの感染死は除外?
(2)37日から92日で既感染者の感染死は除外?
(3)2回目投与から7日までの感染死は除外?
(4)1回目接種後の感染死は除外?
(5)92日以降は未カウント?classic.clinicaltrials.gov/ct2/show/NCT05…


論文の補足資料を見ると、1日目~35日目までのCOVID-19の感染死は0人でした。一方、36日目~92日目では10人になっています。つまり、ワクチン接種者での感染死1名と、プラセボ接種者での感染死9名が36日目~92日目の間で発生したことになります。
デルタ株が流行していたようですが、1日目~35日目までの感染死が0人である理由が、本当に0人であったのか、定義上の理由で0人であったのかまでは不明です。
論文補足資料
static-content.springer.com/esm/art%3A10.1…
デルタ株が流行していたようですが、1日目~35日目までの感染死が0人である理由が、本当に0人であったのか、定義上の理由で0人であったのかまでは不明です。
論文補足資料
static-content.springer.com/esm/art%3A10.1…

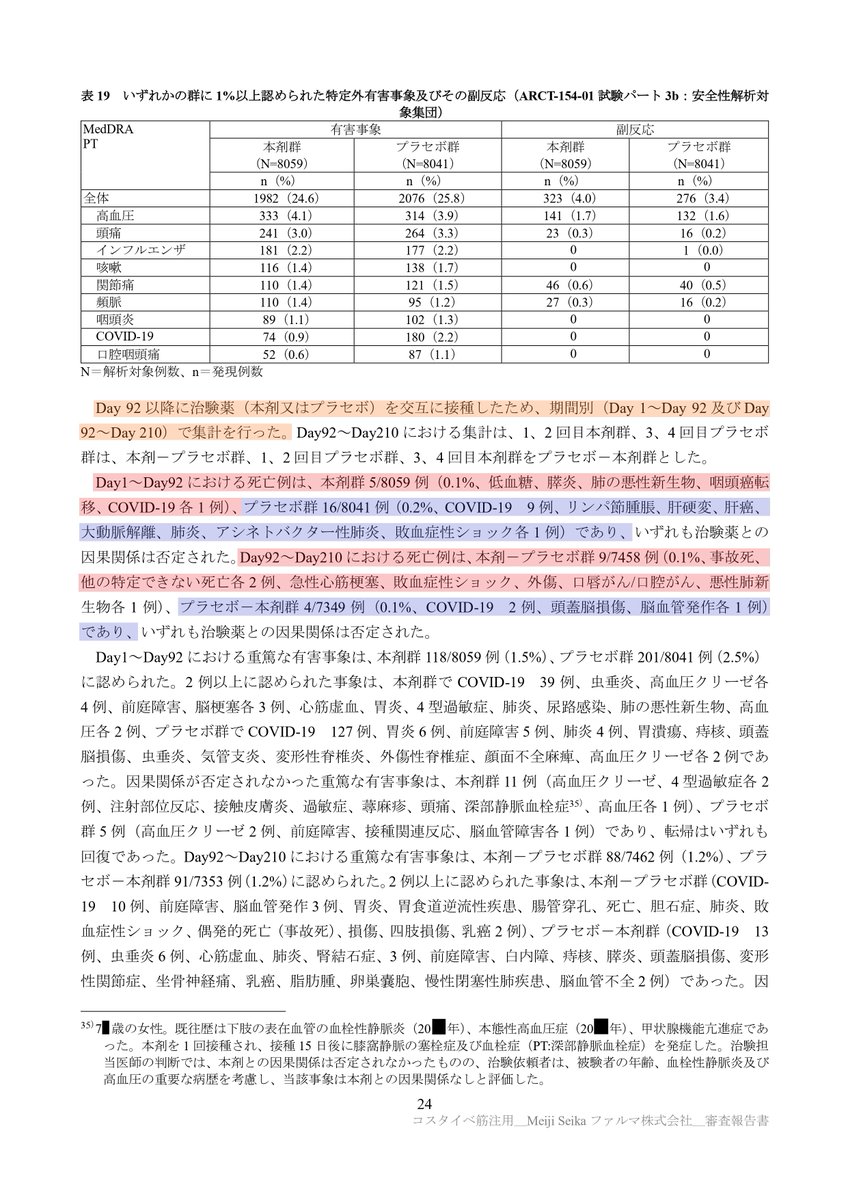
PMDAの資料を見ると、死因の内訳が記載されています。
◆1日目~92日目における死亡例◆
(1)ワクチン接種群:5/8059 例(=0.1%)
低血糖、膵炎、肺の悪性新生物、咽頭癌転移、COVID-19 各1例
(2)プラセボ接種群:16/8041 例(0.2%)
COVID-19(9例)、リンパ節腫脹、肝硬変、肝癌、大動脈解離、肺炎、アシネトバクター性肺炎、敗血症性ショック(各1例)
ここでの死亡者数とCOVID-19感染者数は、論文に開示された数値と一致しています。ここでは、1日目~92日目と記載されていますが、COVID-19感染死の定義は36日目~92日目の間の既感染者の死亡以外の感染死なのではないかと思われます。
◆92日目~210日目における死亡例◆
(1)ワクチン2回→プラセボ2回接種群:9/7458 例(0.1%)
事故死、他の特定できない死亡(各2例)、急性心筋梗塞、敗血症性ショック、外傷、口唇がん/口腔がん、悪性肺新生物(各1例)
(2)プラセボ2回→ワクチン2回接種群:4/7349 例(0.1%)
COVID-19(2例)、頭蓋脳損傷、脳血管発作(各1例)
ここの210日目は120日目の間違いでしょうか?
現時点では、COVID-19の感染死の定義の部分に疑問があります。上述の死亡例に除外された感染死者が含まれているのか、いないのかは不明です。
以上になります。私の記述に囚われず、何かしらの調査のご参考になればと思います。
◆1日目~92日目における死亡例◆
(1)ワクチン接種群:5/8059 例(=0.1%)
低血糖、膵炎、肺の悪性新生物、咽頭癌転移、COVID-19 各1例
(2)プラセボ接種群:16/8041 例(0.2%)
COVID-19(9例)、リンパ節腫脹、肝硬変、肝癌、大動脈解離、肺炎、アシネトバクター性肺炎、敗血症性ショック(各1例)
ここでの死亡者数とCOVID-19感染者数は、論文に開示された数値と一致しています。ここでは、1日目~92日目と記載されていますが、COVID-19感染死の定義は36日目~92日目の間の既感染者の死亡以外の感染死なのではないかと思われます。
◆92日目~210日目における死亡例◆
(1)ワクチン2回→プラセボ2回接種群:9/7458 例(0.1%)
事故死、他の特定できない死亡(各2例)、急性心筋梗塞、敗血症性ショック、外傷、口唇がん/口腔がん、悪性肺新生物(各1例)
(2)プラセボ2回→ワクチン2回接種群:4/7349 例(0.1%)
COVID-19(2例)、頭蓋脳損傷、脳血管発作(各1例)
ここの210日目は120日目の間違いでしょうか?
現時点では、COVID-19の感染死の定義の部分に疑問があります。上述の死亡例に除外された感染死者が含まれているのか、いないのかは不明です。
以上になります。私の記述に囚われず、何かしらの調査のご参考になればと思います。
• • •
Missing some Tweet in this thread? You can try to
force a refresh