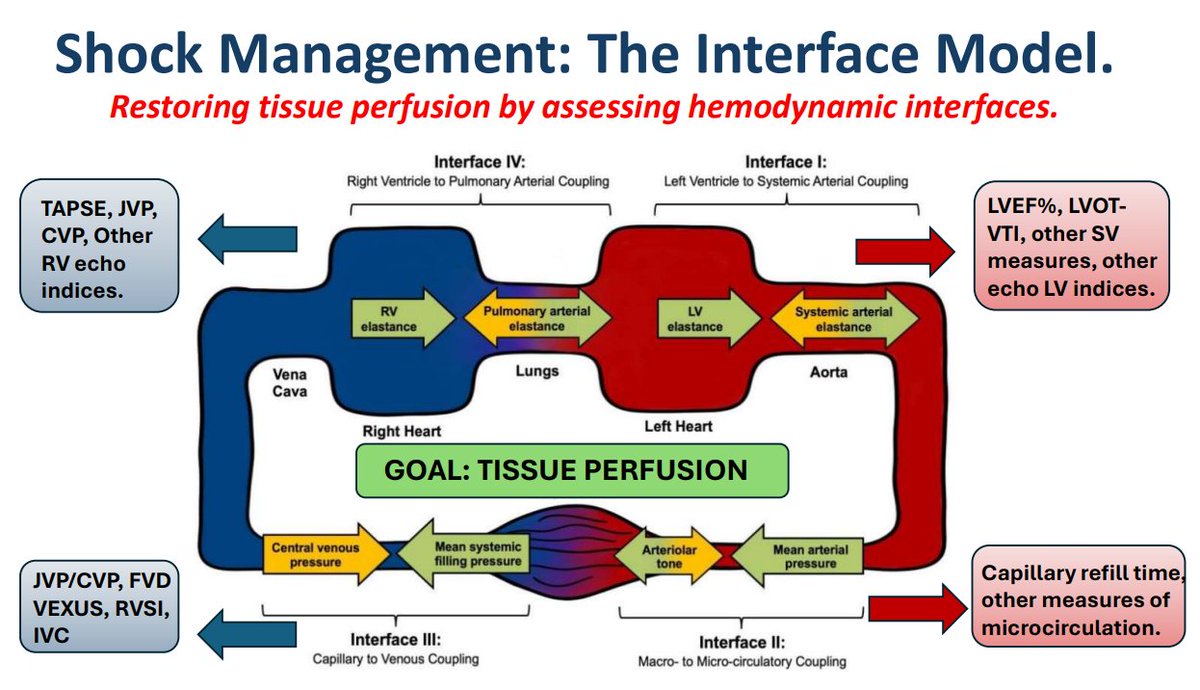
🧵 "What really determines tissue perfusion?"
– and why most explanations get it wrong.
Let’s sort out MAP, CVP, CCP, autoregulation, vasopressors, and the flow that actually reaches your organs.
👇
– and why most explanations get it wrong.
Let’s sort out MAP, CVP, CCP, autoregulation, vasopressors, and the flow that actually reaches your organs.
👇

1/
You’ve probably heard:
“Perfusion pressure = MAP − CVP”
Or sometimes:
“Perfusion = MAP − CCP”
But both are context-dependent.
Let’s unpack what truly drives tissue perfusion — and why it’s more dynamic than most realise.
You’ve probably heard:
“Perfusion pressure = MAP − CVP”
Or sometimes:
“Perfusion = MAP − CCP”
But both are context-dependent.
Let’s unpack what truly drives tissue perfusion — and why it’s more dynamic than most realise.
3/
The simplest model:
Flow = (MAP − Venous Pressure) / Resistance
Where:
MAP = pressure in
Venous pressure = pressure out
Resistance = mostly arteriolar tone
Simple. But misleading.
The simplest model:
Flow = (MAP − Venous Pressure) / Resistance
Where:
MAP = pressure in
Venous pressure = pressure out
Resistance = mostly arteriolar tone
Simple. But misleading.
4/
The problem?
🧠 Resistance isn’t fixed.
Tissues actively adjust arteriolar tone to preserve flow — even when MAP changes.
This is called autoregulation.
The problem?
🧠 Resistance isn’t fixed.
Tissues actively adjust arteriolar tone to preserve flow — even when MAP changes.
This is called autoregulation.
5/
Autoregulation allows tissues to keep flow constant over a range of MAP values.
If MAP drops → arterioles dilate
If MAP rises → arterioles constrict
The goal is to preserve capillary flow, despite pressure changes upstream.
Autoregulation allows tissues to keep flow constant over a range of MAP values.
If MAP drops → arterioles dilate
If MAP rises → arterioles constrict
The goal is to preserve capillary flow, despite pressure changes upstream.
6/
So within that range, flow stays stable even when the driving pressure (MAP) changes.
The tissue is controlling its own flow.
And it doesn’t care what’s happening downstream — as long as venous pressures are low.
So within that range, flow stays stable even when the driving pressure (MAP) changes.
The tissue is controlling its own flow.
And it doesn’t care what’s happening downstream — as long as venous pressures are low.
7/
But autoregulation has limits.
If:
• MAP falls below the lower threshold
• Arterioles can’t dilate further
Then flow begins to fall — it’s now pressure-dependent.
🧠 Autoregulation can’t help if the input pressure is too low.
But autoregulation has limits.
If:
• MAP falls below the lower threshold
• Arterioles can’t dilate further
Then flow begins to fall — it’s now pressure-dependent.
🧠 Autoregulation can’t help if the input pressure is too low.
8/
So what about venous pressure (e.g. CVP)?
It doesn’t trigger autoregulation.
It just quietly opposes flow.
And when it rises, it narrows the perfusion gradient — with no compensatory response.
In this setting, venous pressure becomes the key limiter of flow.
So what about venous pressure (e.g. CVP)?
It doesn’t trigger autoregulation.
It just quietly opposes flow.
And when it rises, it narrows the perfusion gradient — with no compensatory response.
In this setting, venous pressure becomes the key limiter of flow.
9/
That’s why venous congestion is dangerous.
You can have a “normal” MAP but still under-perfuse tissues as the pressure gradient drops.
And there’s no mechanism to compensate
Flow falls — silently.
That’s why venous congestion is dangerous.
You can have a “normal” MAP but still under-perfuse tissues as the pressure gradient drops.
And there’s no mechanism to compensate
Flow falls — silently.
10/
Now let’s add another piece:
🩸 Critical Closing Pressure (CCP)
This is the pressure below which a vessel collapses and flow stops, even if venous pressure is lower.
It reflects:
• Vascular tone
• External tissue pressure
Now let’s add another piece:
🩸 Critical Closing Pressure (CCP)
This is the pressure below which a vessel collapses and flow stops, even if venous pressure is lower.
It reflects:
• Vascular tone
• External tissue pressure
11/
If vascular tone is very high (e.g. excessive vasoconstriction), or external pressure is elevated (e.g. brain swelling, oedema, compartment syndrome), CCP rises.
Now, even if MAP is "normal", there’s no flow unless it's above CCP.
If vascular tone is very high (e.g. excessive vasoconstriction), or external pressure is elevated (e.g. brain swelling, oedema, compartment syndrome), CCP rises.
Now, even if MAP is "normal", there’s no flow unless it's above CCP.
12/
This is the vascular waterfall.
When vessels collapse like a choke point, flow becomes:
Flow = (MAP − CCP) / Resistance
CVP no longer matters — the collapsed segment sets the outflow pressure.
This is the vascular waterfall.
When vessels collapse like a choke point, flow becomes:
Flow = (MAP − CCP) / Resistance
CVP no longer matters — the collapsed segment sets the outflow pressure.
13/
So when does CCP matter?
• In high tone states
• With external compression (e.g. raised ICP)
• And potentially with overuse of vasopressors, which can raise CCP via excessive arteriolar constriction
So when does CCP matter?
• In high tone states
• With external compression (e.g. raised ICP)
• And potentially with overuse of vasopressors, which can raise CCP via excessive arteriolar constriction
14/
So what really determines tissue perfusion?
It’s not MAP alone.
Not MAP − CVP.
Not MAP − CCP.
It’s how...
• MAP
• Venous pressure
• Resistance
• CCP
...all interact
And how the tissue responds (or can’t).
So what really determines tissue perfusion?
It’s not MAP alone.
Not MAP − CVP.
Not MAP − CCP.
It’s how...
• MAP
• Venous pressure
• Resistance
• CCP
...all interact
And how the tissue responds (or can’t).
15/
So beware simple formulas.
Perfusion isn’t about plugging numbers into a neat equation.
It’s about context — autoregulation, tone, congestion, and where the choke points lie.
And understanding that changes how you manage shock, fluids, and pressors.
So beware simple formulas.
Perfusion isn’t about plugging numbers into a neat equation.
It’s about context — autoregulation, tone, congestion, and where the choke points lie.
And understanding that changes how you manage shock, fluids, and pressors.
16/
Coming soon:
🔹 A full thread on Critical Closing Pressure
🔹 How vasopressors, tone & compression affect it
🔹 Why MAP alone doesn’t guarantee flow
Follow to catch it.
#MedX #CriticalCare #Physiology #Haemodynamics
Coming soon:
🔹 A full thread on Critical Closing Pressure
🔹 How vasopressors, tone & compression affect it
🔹 Why MAP alone doesn’t guarantee flow
Follow to catch it.
#MedX #CriticalCare #Physiology #Haemodynamics
Start here ⬇️
https://x.com/icmteaching/status/1949502195965083941
• • •
Missing some Tweet in this thread? You can try to
force a refresh