
https://t.co/s5bd9qxm2e. Associate Prof of Biostatistics at @EmoryRollins, @EmoryBIOS. Co-director @EmoryEAVE.
23 subscribers
How to get URL link on X (Twitter) App


https://twitter.com/DataSciJedi/status/1554512848520417287Our first speaker is @ajrgodfrey. He speaks from his experiences as a blind person. He emphasizes the importance of independence and dignity for the visually impaired. #JEDIatJSM

 Imagine a variant with little capacity for re-infection. The susceptible population is exposed to enough virus to infect. The infections include severe, moderate, mild disease, and asymptomatic infections. (Here I point out that these sketches are not to scale.) 2/8
Imagine a variant with little capacity for re-infection. The susceptible population is exposed to enough virus to infect. The infections include severe, moderate, mild disease, and asymptomatic infections. (Here I point out that these sketches are not to scale.) 2/8 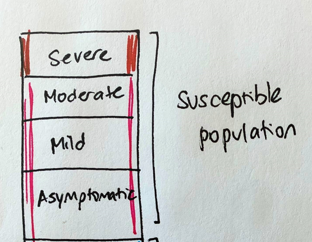

https://twitter.com/USMortality/status/1443431592249094146In a test negative study, we only recruit people who develop symptoms. PCR is then used to distinguish positive cases from negative controls (people with symptoms, but due to another cause). COVID vaccines are not expected to prevent other causes of symptoms, like a cold.
https://twitter.com/nataliexdean/status/1427703094062706691?s=20

 Using data from the Moderna trial, scientists studied the relationship between post-vaccination antibody levels and protection against disease. This is a unique study design to link the antibody response to efficacy directly.
Using data from the Moderna trial, scientists studied the relationship between post-vaccination antibody levels and protection against disease. This is a unique study design to link the antibody response to efficacy directly.

 First, some definitions. We are familiar with vaccine efficacy (VE) against disease. This was estimated from trials. But many studies have helped us estimate VE against all infection (aka VE_S). For mRNA vaccines against early strains, this has hovered around 80-90%. 2/11
First, some definitions. We are familiar with vaccine efficacy (VE) against disease. This was estimated from trials. But many studies have helped us estimate VE against all infection (aka VE_S). For mRNA vaccines against early strains, this has hovered around 80-90%. 2/11
https://twitter.com/novavax/status/1404379473085091843The two dose protein subunit vaccine can be stored at refrigerated temperatures and is cheaper to produce. The company will apply for an EUA in the third quarter of 2021, as they need more time to validate the manufacturing process.
https://twitter.com/JenniferNuzzo/status/1400546130279211013There's a lot we can learn from this experience about:
https://twitter.com/ErinBromage/status/1394692831575486470?s=20

 He provides a successful example of three large-scale platform trials collaborating to harmonize protocols for antithrombotics. Data are more valuable when they can be combined and compared. 2/4
He provides a successful example of three large-scale platform trials collaborating to harmonize protocols for antithrombotics. Data are more valuable when they can be combined and compared. 2/4 