Intriguing article in the June issue of @JTOonline by #JongWooLee and @DrRoyHerbstYale tackling the impact of #KRAS on the immune microenvironment in #NSCLC and the rationale for combining #MEK inhibition and anti-PD(L)1 therapy. #OncoAlert #LCSM
jto.org/article/S1556-…
jto.org/article/S1556-…
Authors induced a p53/KRAS G12D mutant NSCLC. Compared to tumor free lungs, see a stark increase in CD45+ cells. Note, geographically localized to periphery/margin. Translational challenge: easy to miss with biopsies(aspirates not helpful). 
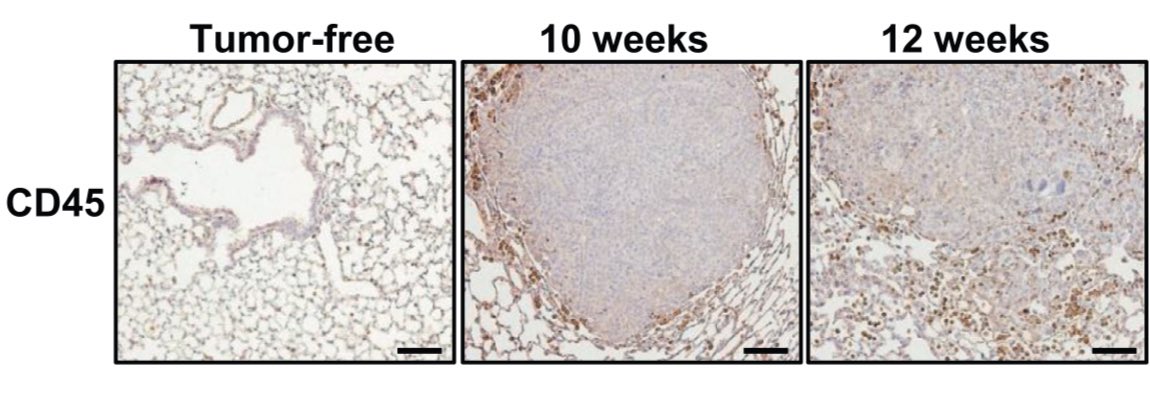
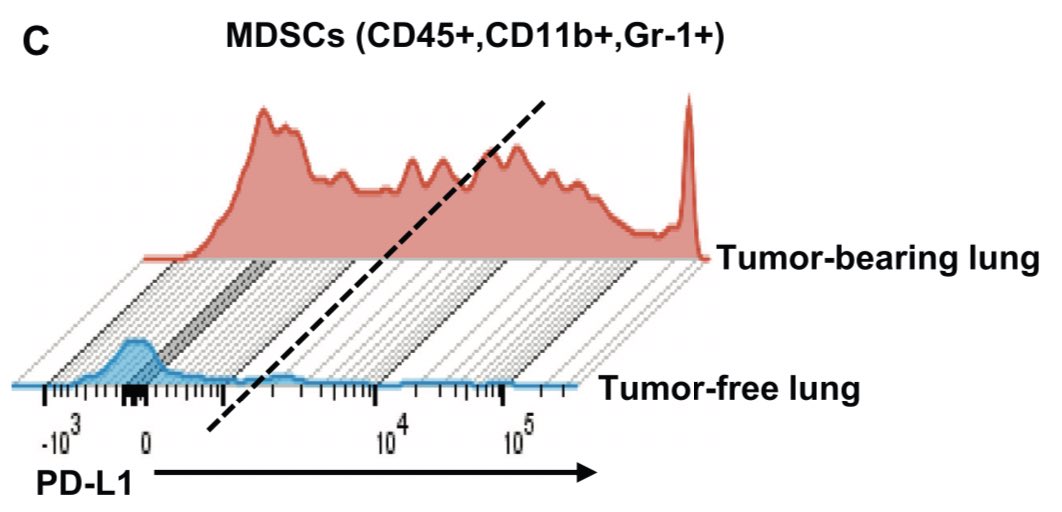
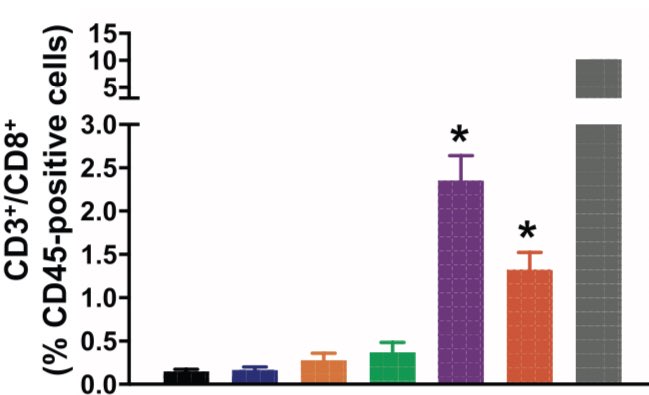
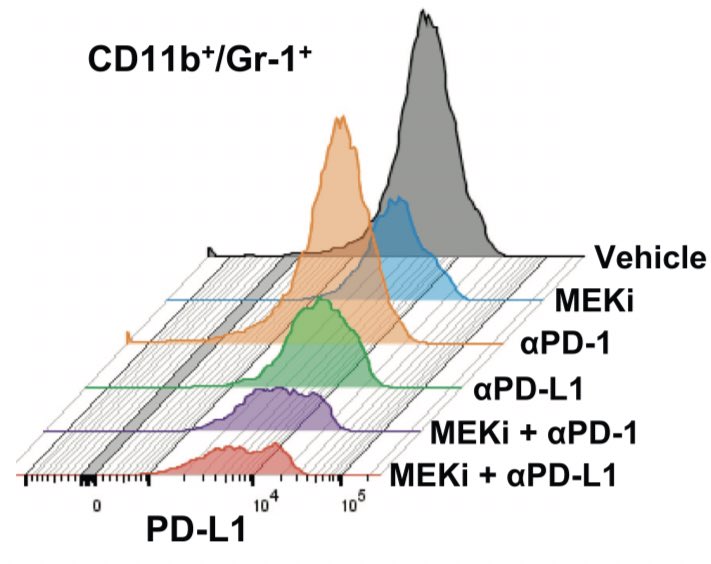
Specifically, there’s a rise in neutrophils and MDSCs and a decrease in CD3+/CD8+ T cells in the tumor bearing lung (red bars below). Accompanied by rise in PDL1 expression (in tumor and MDSCs). Story: does this immune cell accumulation precede tumor development? Is it required? 






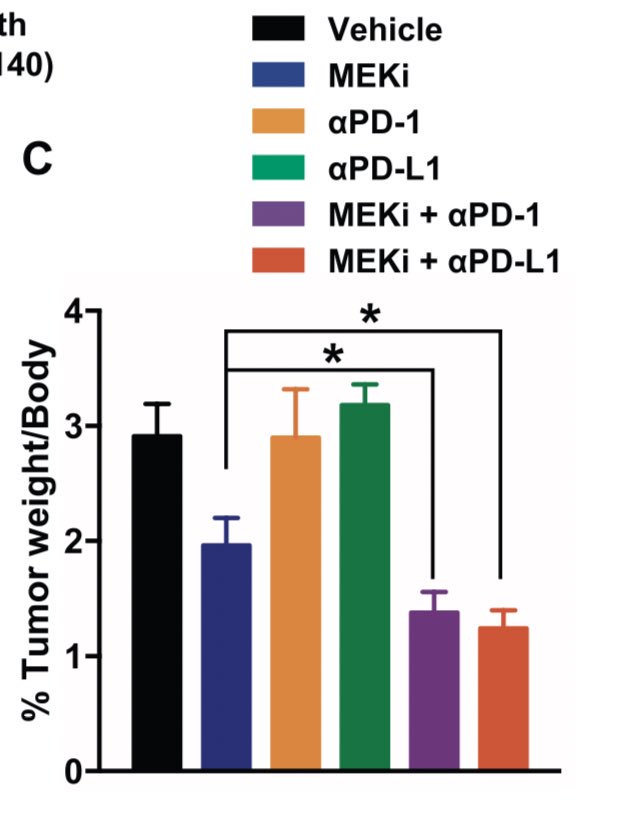
In this transgenic mouse model, use of PD(L)1 monotherapy ineffective (orange and green bars). #MEK inhibition led to response (blue). We can see this clinically but typically transient. Combination of MEKi and PD(L)1 led to greatest decrease (purple and red bars). 

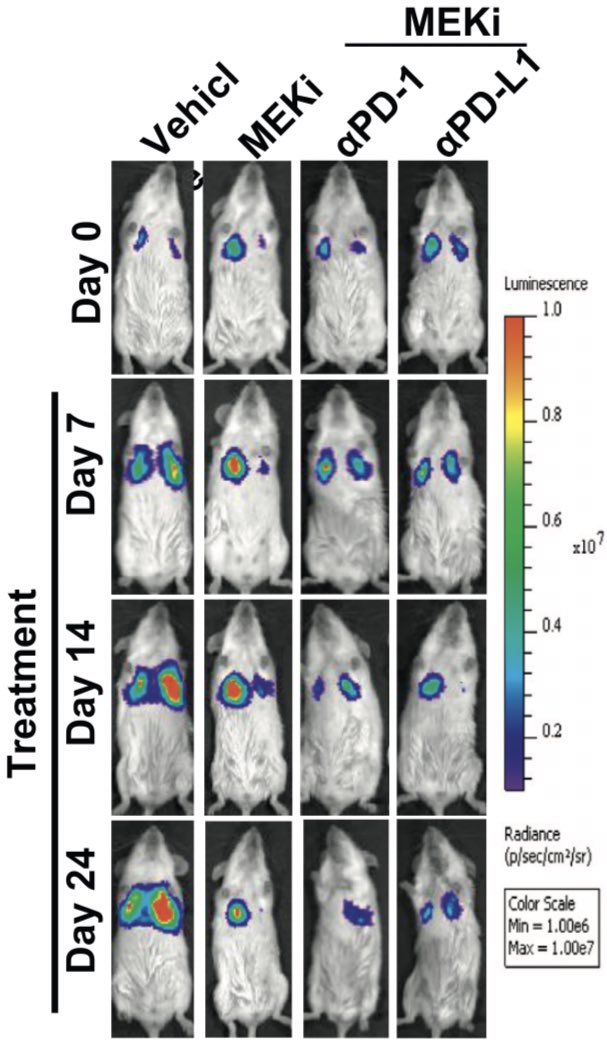
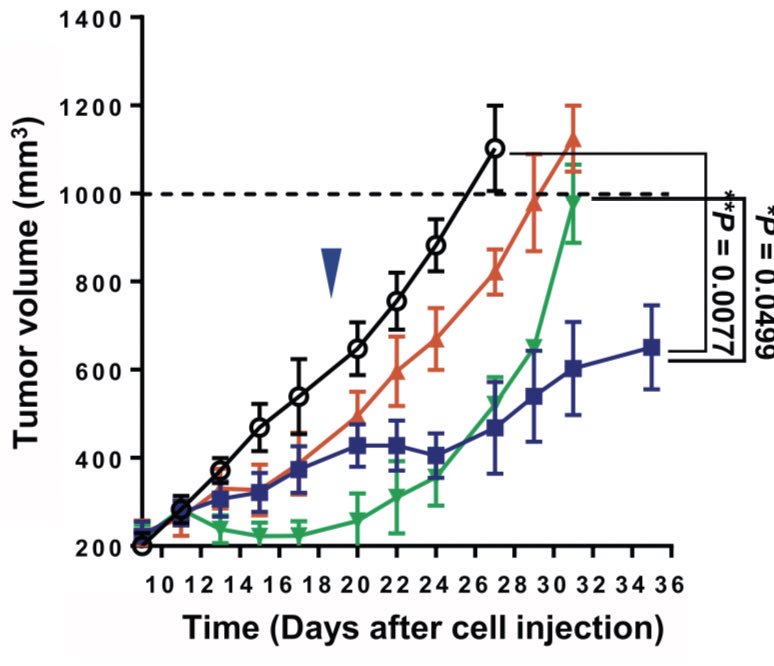
Combination of MEK inhibition and anti-PD(L)1 then explored in immunocompetent syngeneic mouse model. KRAS tumors implanted and treated. PD(L)1 alone had no impact. Combination led to response (far right) and longer survival, even with low doses of MEK inhibition (purple line) 


What’s happening here? When analyzed by flow cytometry, combination of MEK inhibition and PDL1 therapy (purple and red) augments CD8+ T cell population AND ablates MDSC population. Also seen: a dramatic decrease in PDL1+ MDSCs after combination therapy. 

In vehicle treated tumors, CD3+ T cells relegated to tumor periphery. In MEK-inhibitor treated tumors, T cells seen infiltrating into the tumor leading to a decrease in proliferation and an increase in apoptosis. 





Possible story: in this KRAS G12D model, combining MEK inhibitor (even at low doses) and PDL1 therapy can increase T cell infiltration into the tumor, decrease MDSCs, and ablate tumor growth.
Combination in colon cancer disappointing but some responses seen. One issue may have been dose and tolerability. These data support lower doses (or aggressive modification) with the same immunomodulatory impact. Consistent with prior preclinical work.
Would encourage #ETCTN colleagues to activate NCI #10166 - atezolizumab and cobimetinib in IO refractory #NSCLC, currently for #KRAS positive tumors. Extensive biomarker studies focused on the #TME should be very informative. #LCSM #ClinicalTrials
clinicaltrials.gov/ct2/show/NCT03…
clinicaltrials.gov/ct2/show/NCT03…
• • •
Missing some Tweet in this thread? You can try to
force a refresh