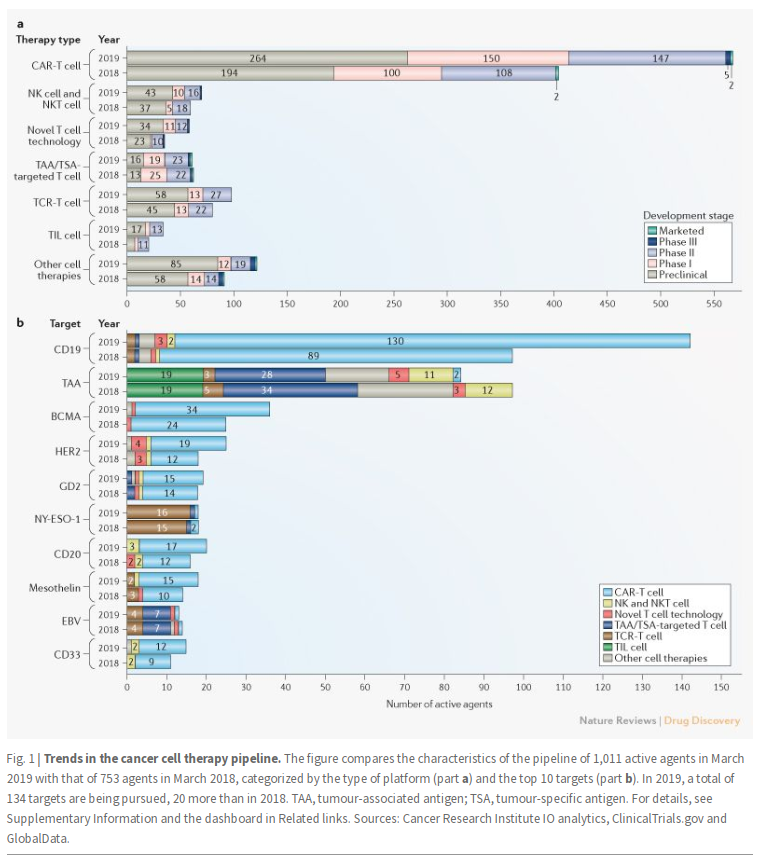
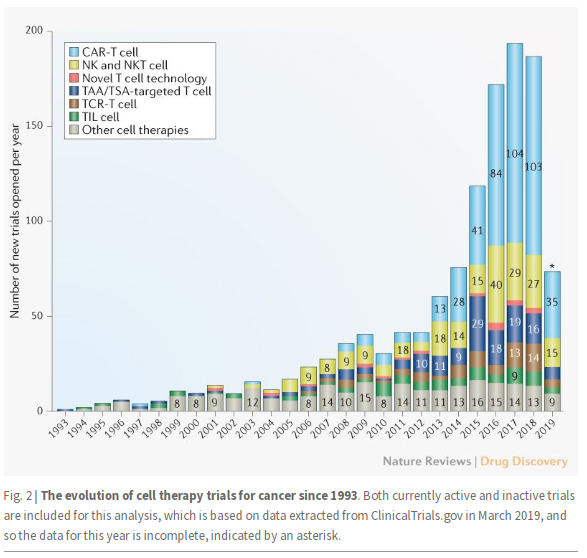
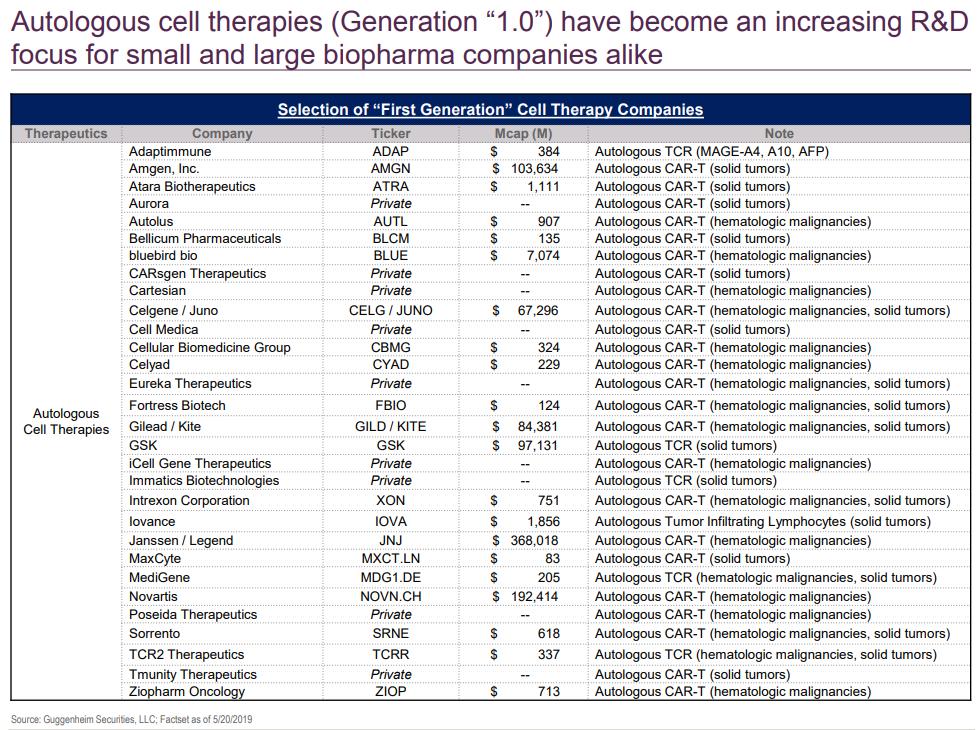
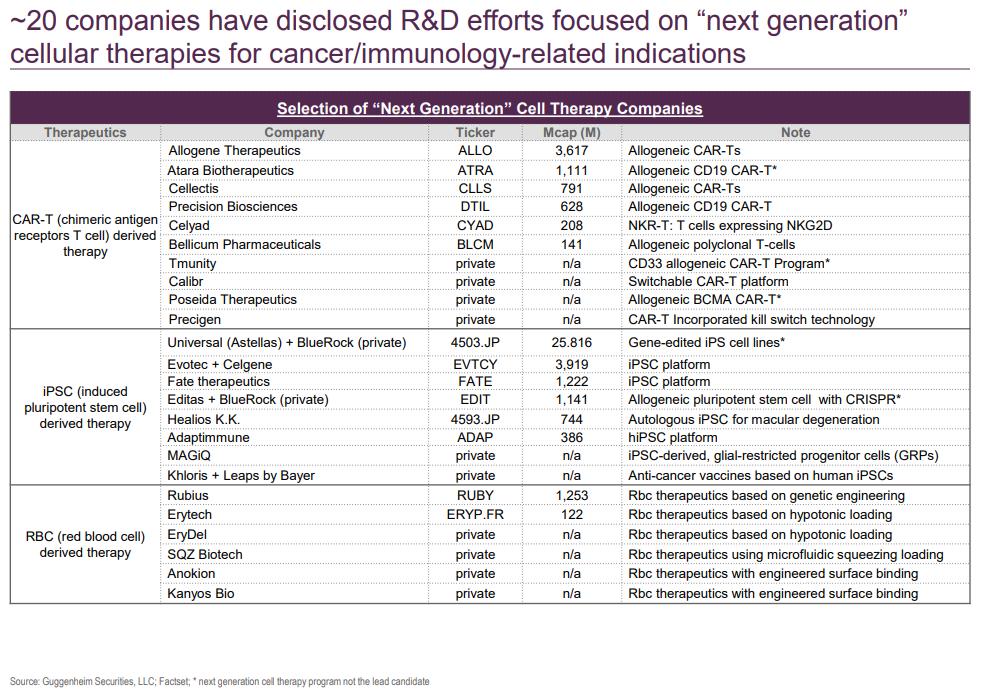
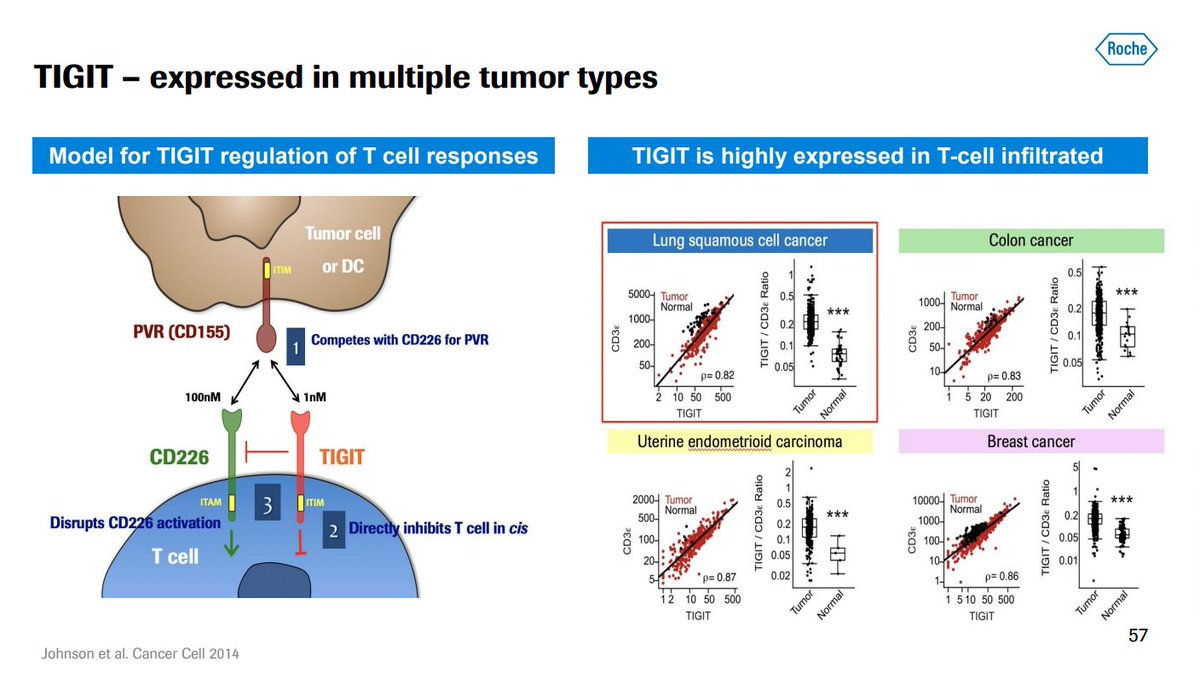
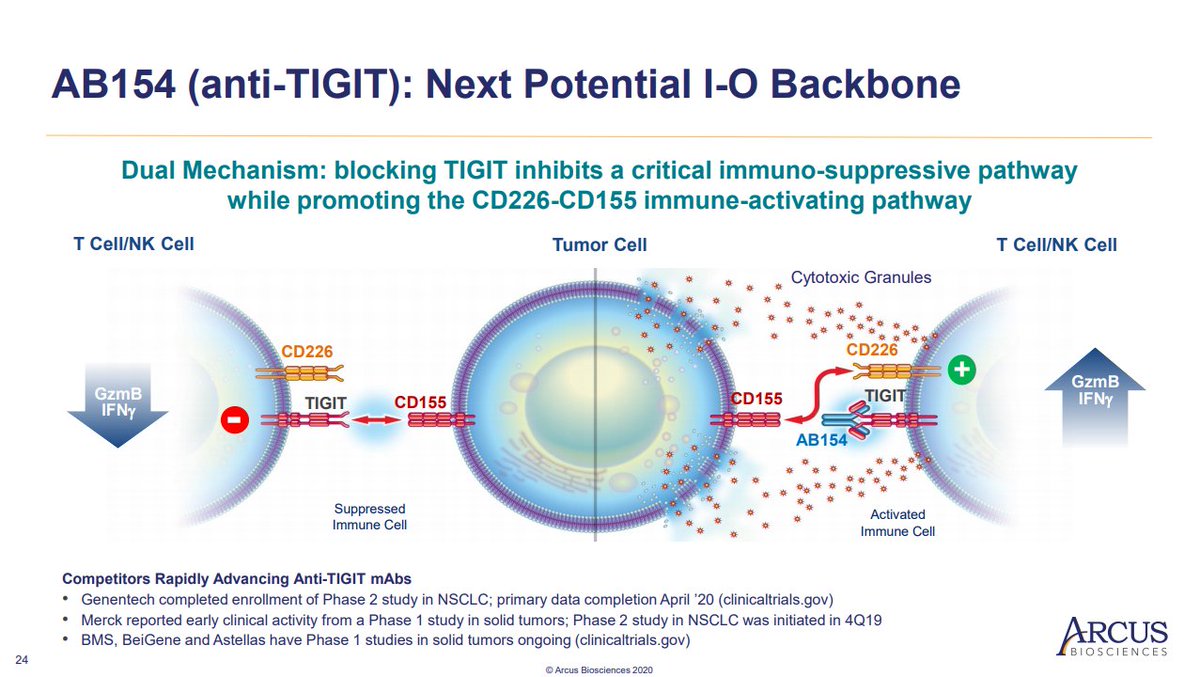
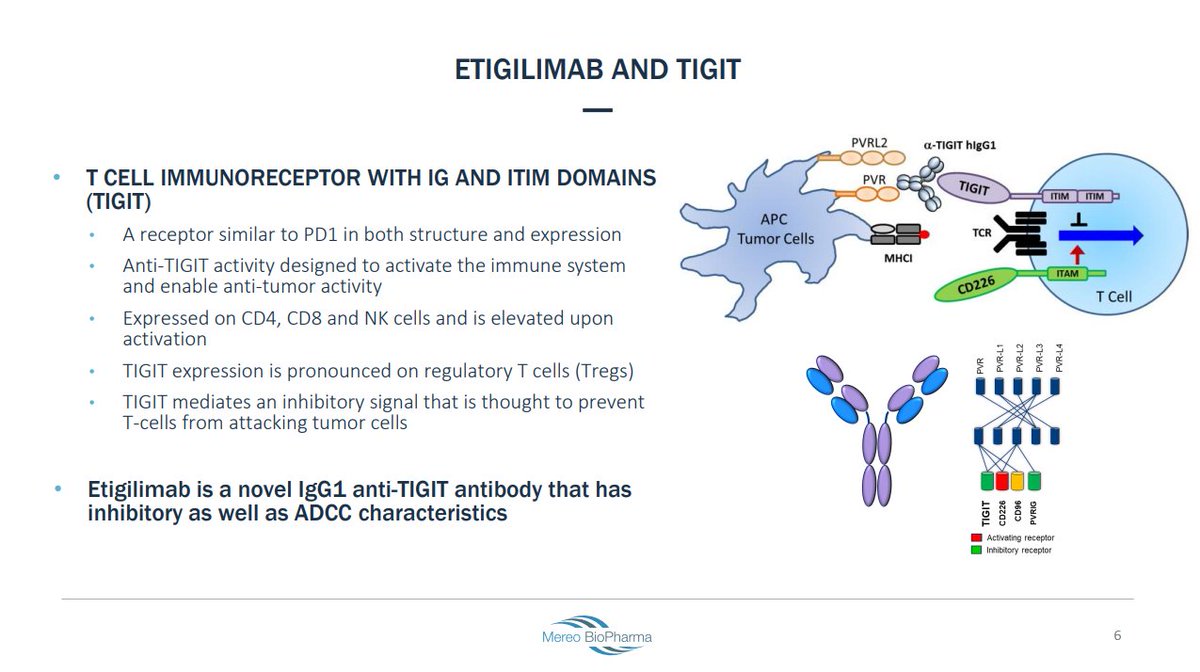
- Ligand for TIGIT (PVR) is expressed in multiple tumor types
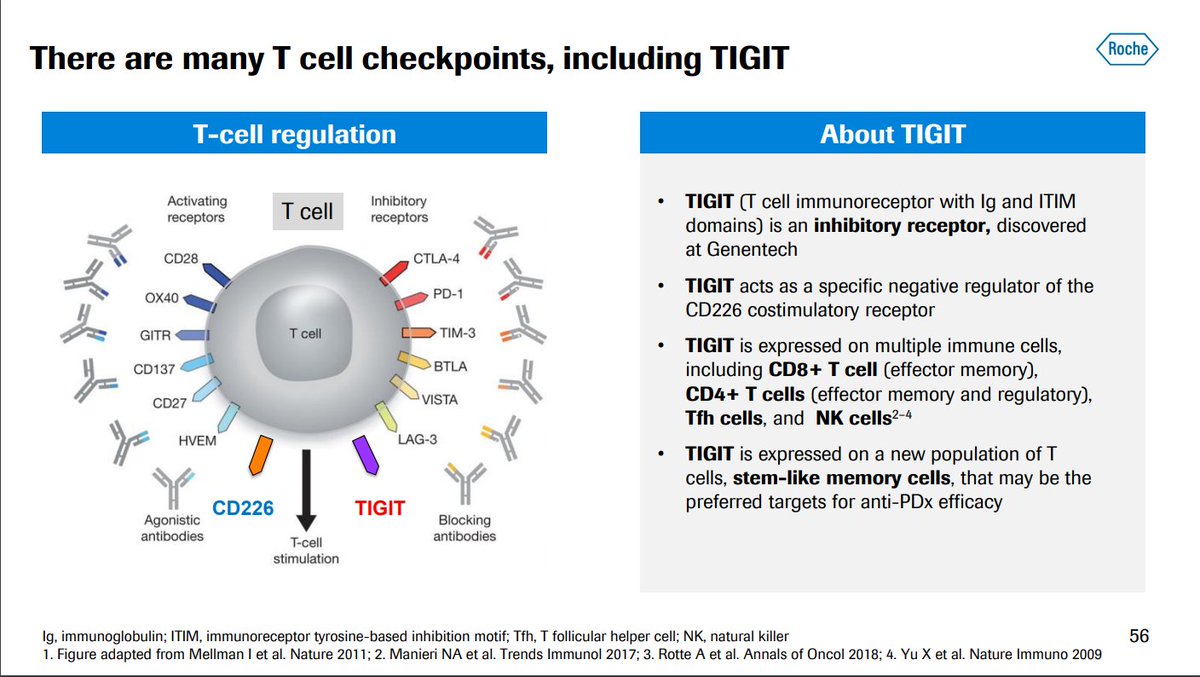
- Activation suppresses T-cell activity
- Upregulated during PD-1 blockade
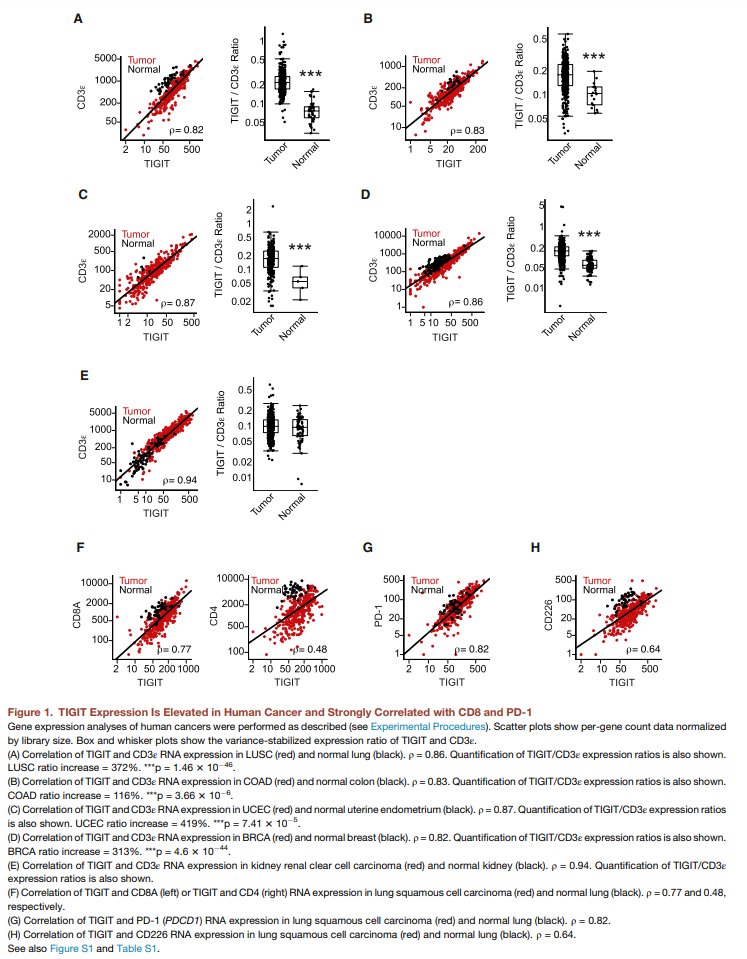
@Cancer_Cell paper (open access): cell.com/cancer-cell/fu…
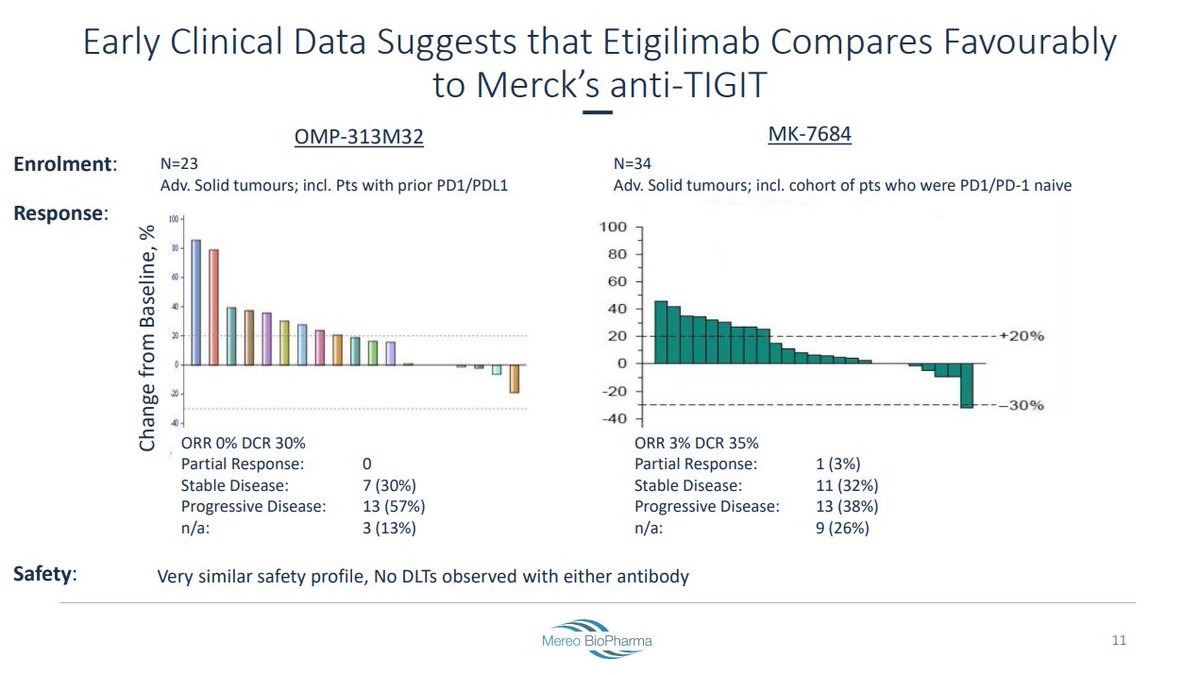
MK-7684 Phase 1 data in advanced solid tumors were presented at #SITC18. No DLTs were observed.
investors.merck.com/news/press-rel…
iTeos is backed by MPM Capital, HBM, 6 Dimensions & Curative Ventures.
@iTeosTx PR: iteostherapeutics.com/news/iteos-the…
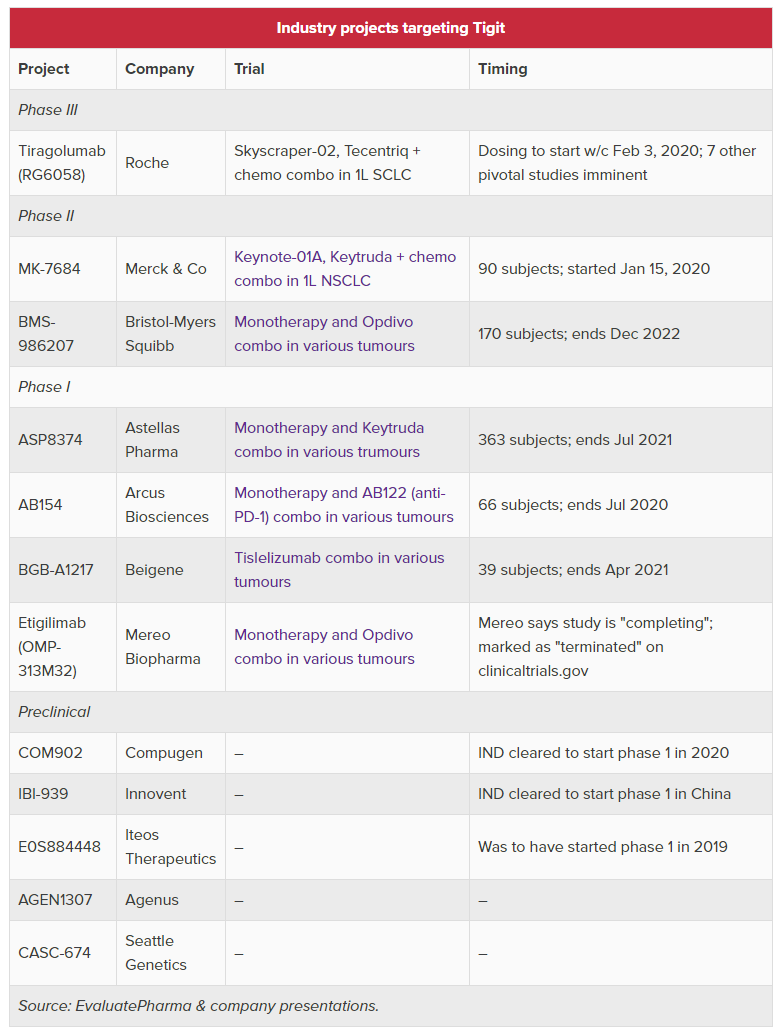
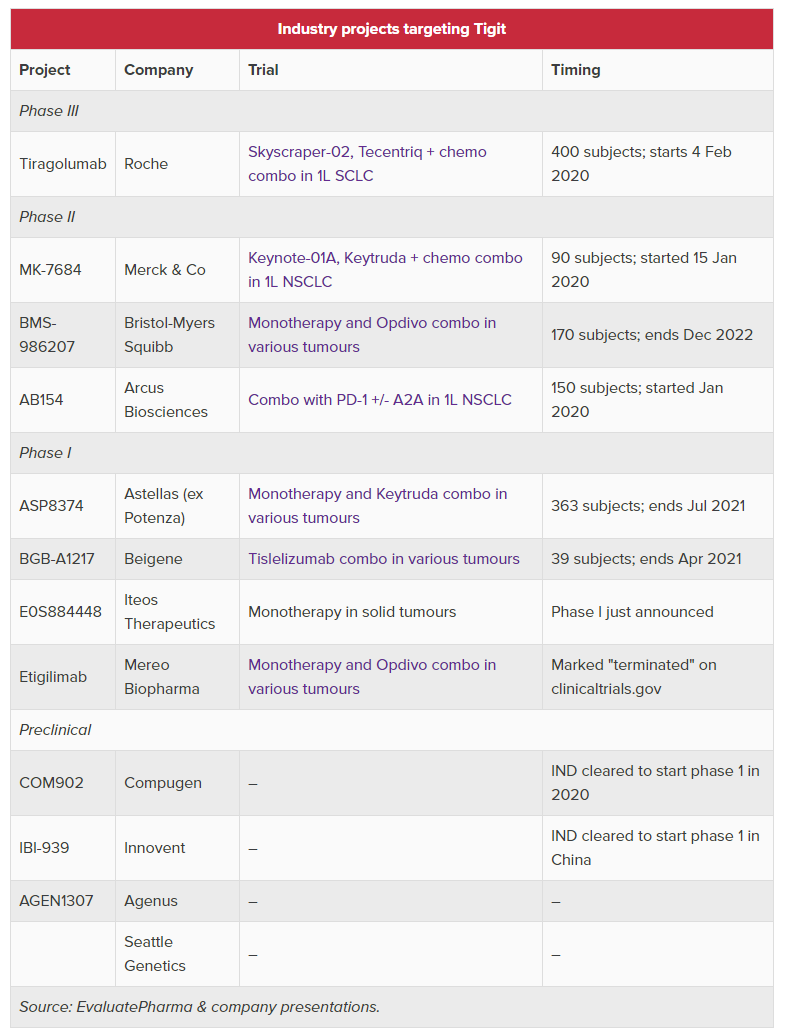
(Updated TIGIT table below)
Mereo gained OncoMed’s etigilimab through their #ReverseMerger last year. Note that $CELG gave back full rights in Jun '19.
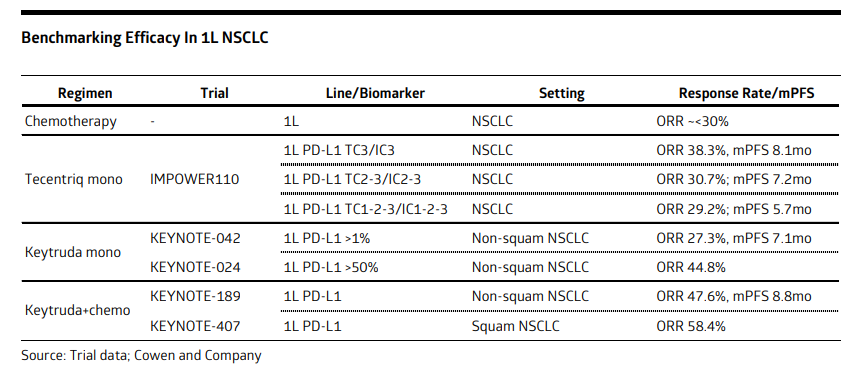
These trials are in 1L #NSCLC, PD-1-refractory #melanoma (+anti-CTLA-4), 1L melanoma and neoadjuvant melanoma.
Great coverage by @JacobPlieth & @Vantageanalysis (see below)
evaluate.com/vantage/articl…
$RHHBY $MRK $BMY $RCUS $CGEN
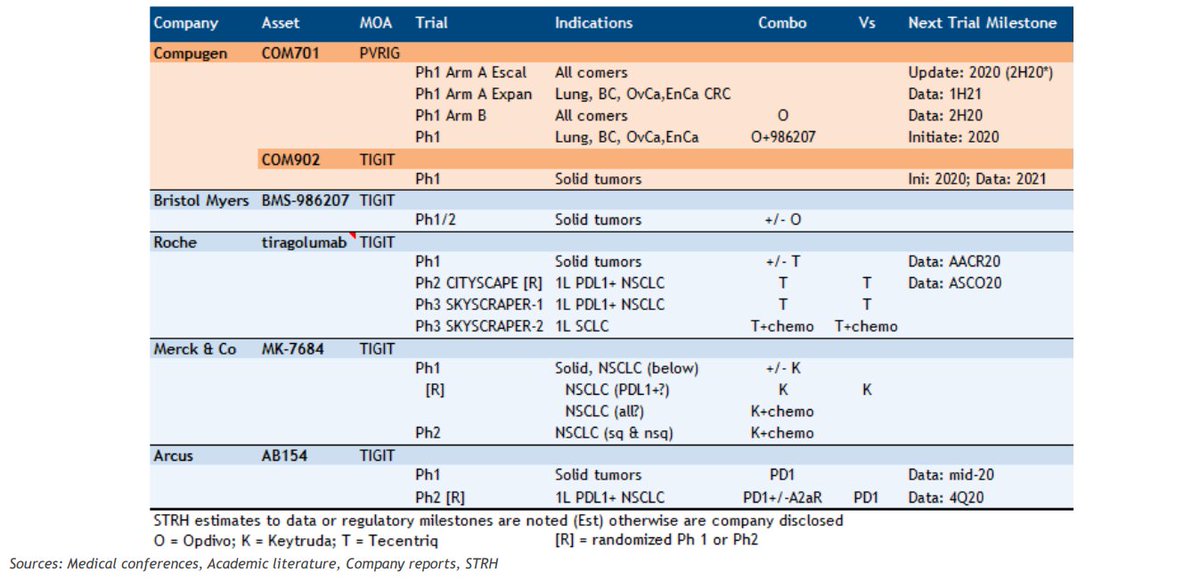
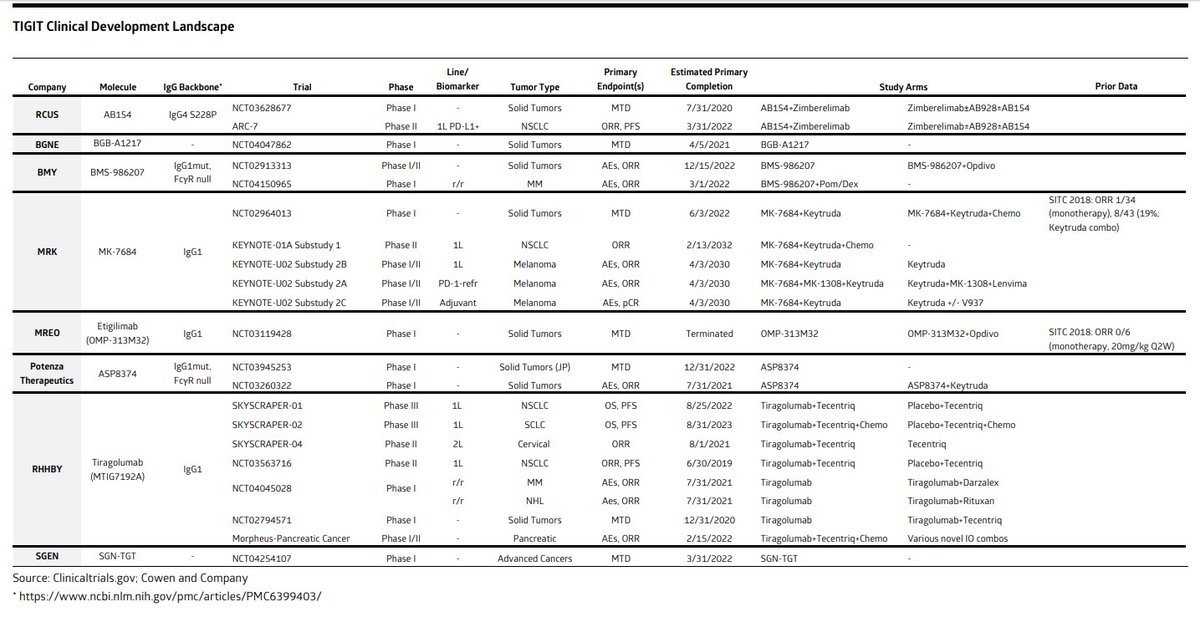
Updated TIGIT landscape below (Cowen). $MRK $BMY $RCUS $BGNE $MREO $SGEN $CGEN $AGEN $IVBXF & Potenza
Interesting that Boxer co-led this deal and recently took a 9.8% stake in $MREO:
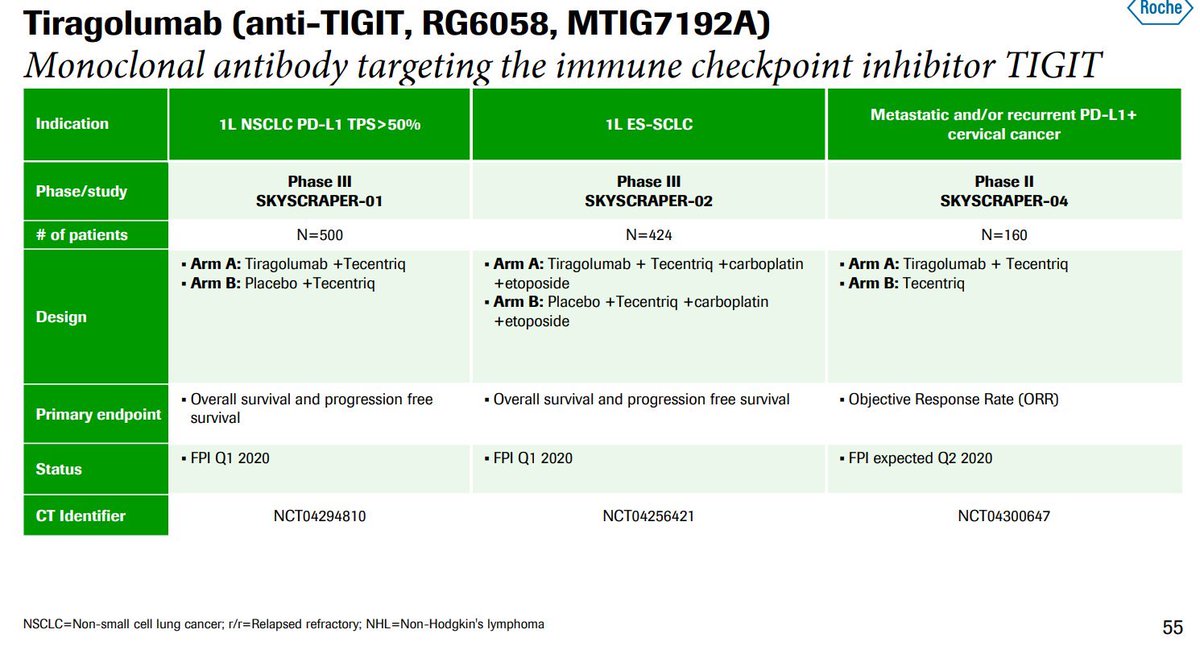
The upcoming Roche tiragolumab data will be telling... $RHHBY
Deck: roche.com/dam/jcr:f5b0e8…
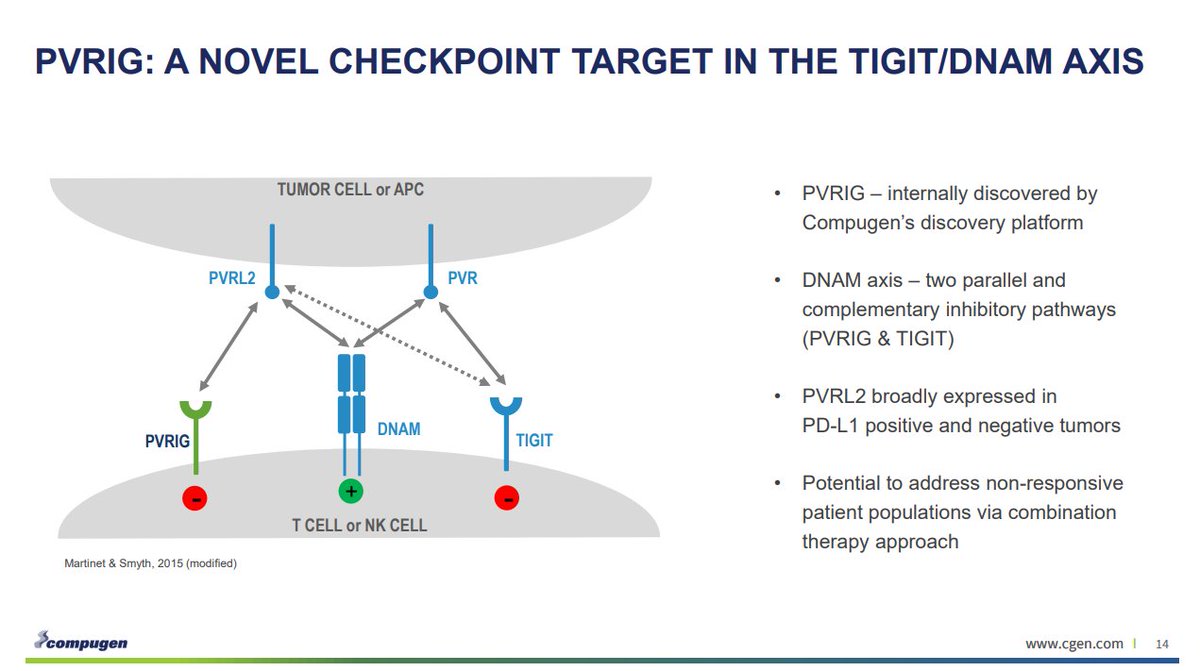
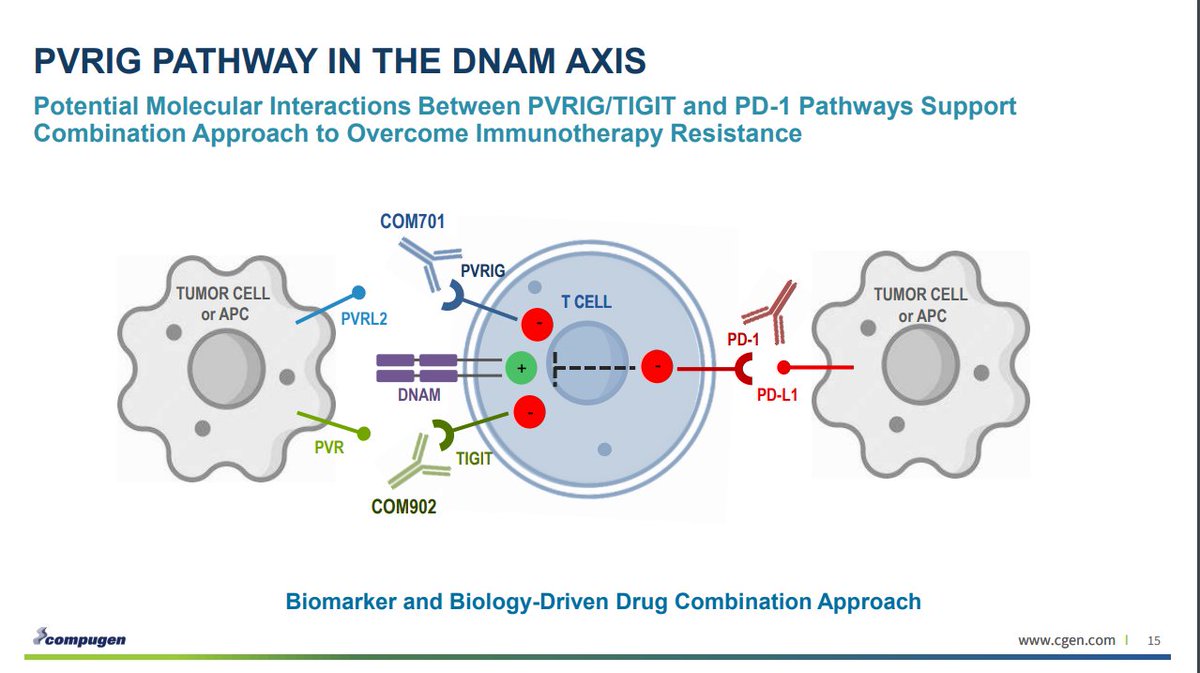
PVRIG is a part of the DNAM axis, along with TIGIT.
@CIR_AACR Feb '19 background paper: cancerimmunolres.aacrjournals.org/content/7/2/257
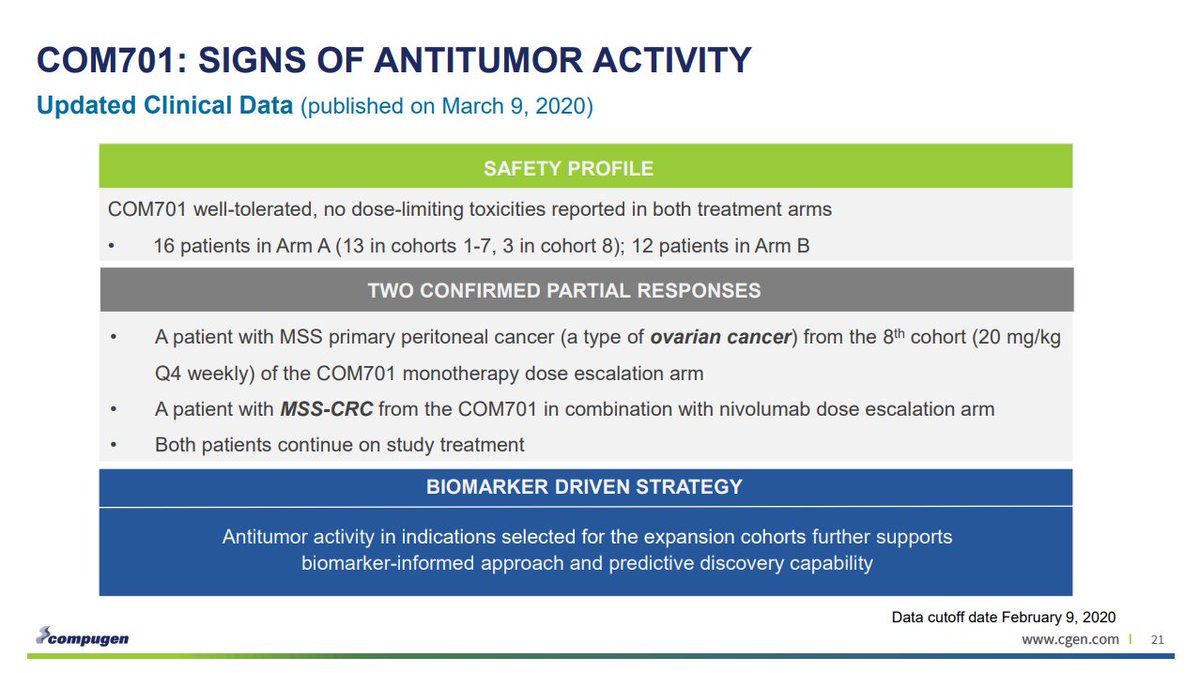
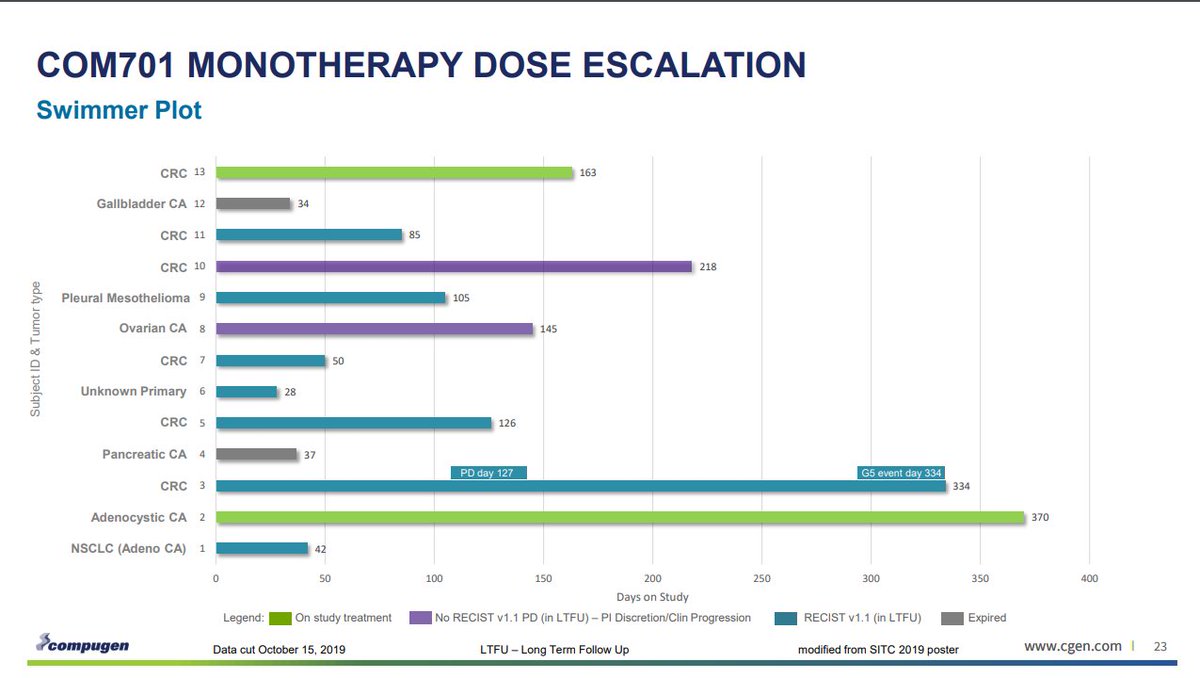
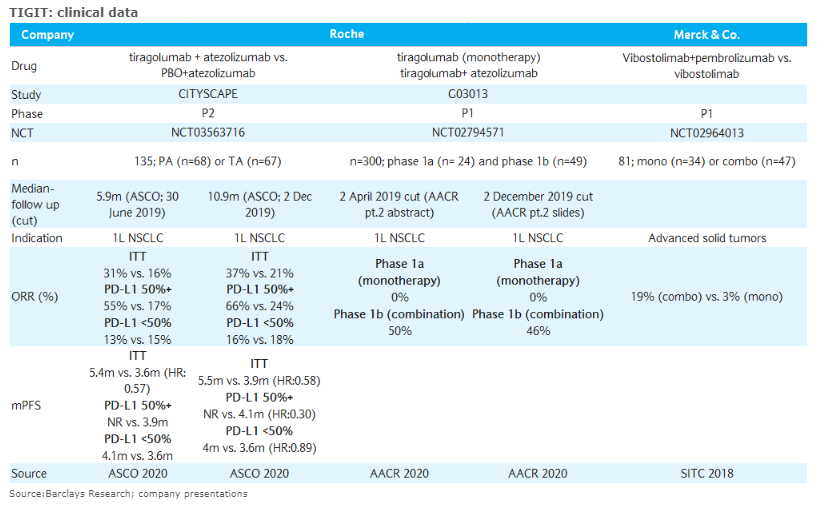
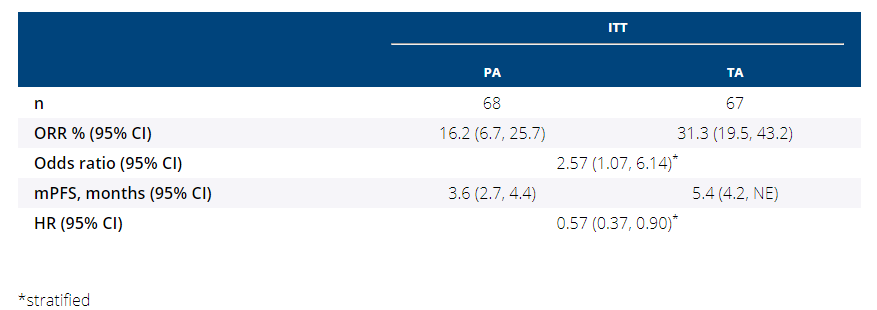
Primary analysis:
- ORR: 31.3% vs 16.2%
- mPFS: 5.4 mo vs 3.6 mo (HR=0.57)
6 mo follow-up analysis:
- ORR: 37.3% vs 20.6%
- mPFS: 5.6 mo vs 3.9 mo
Abstract: meetinglibrary.asco.org/record/184799/…
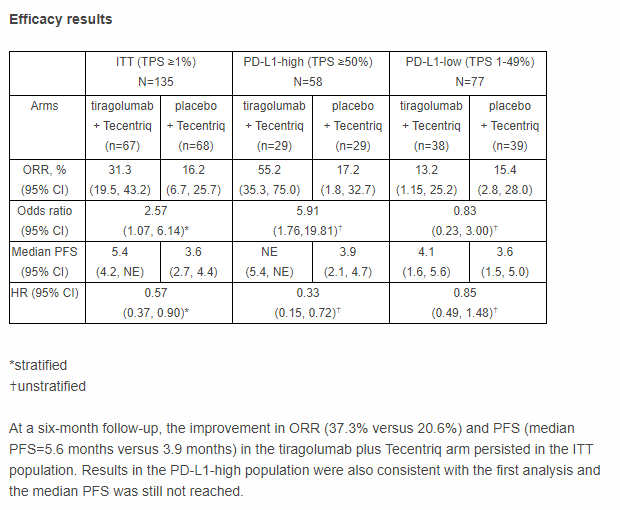
PD-L1-high (TPS ≥50%):
- n=58
- ORR: 55.2% vs 17.2%
- mPFS: Not reached vs 3.6 mo (HR=0.33)
PD-L1-low (TPS 1-49%):
- n=77
- ORR: 13.2% vs 15.4%
- mPFS: 4.1 mo vs 3.6 mo (HR=0.85)
PR: businesswire.com/news/home/2020…
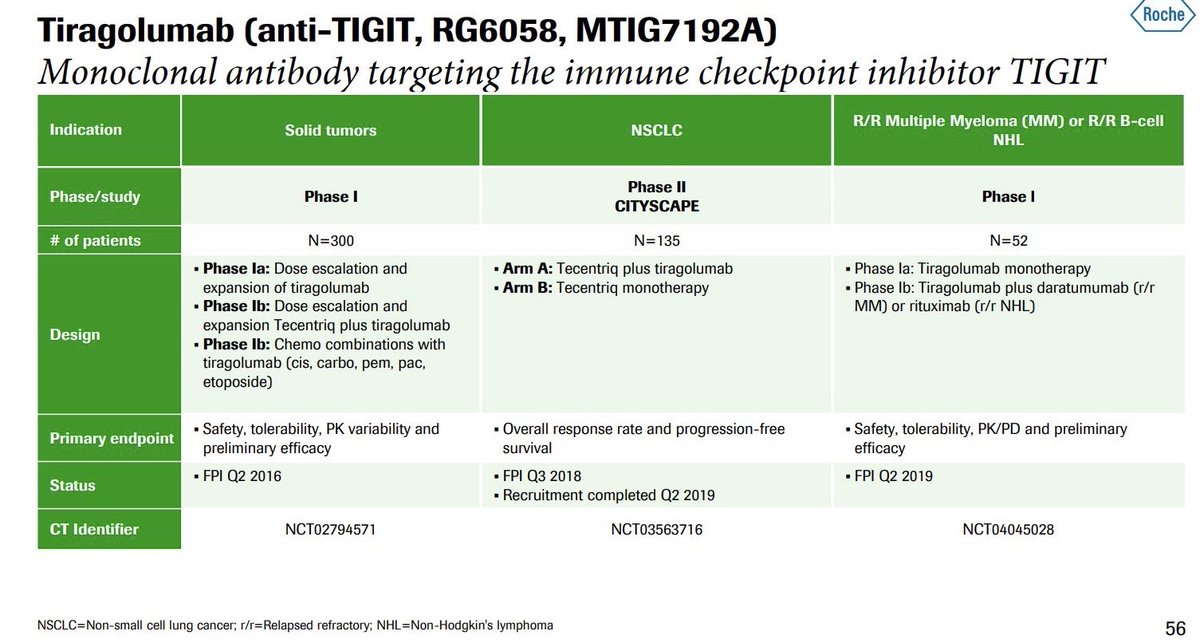
"Phase Ia/Ib dose-escalation study of the anti-TIGIT antibody tiragolumab as a single agent and in combination with atezolizumab in patients with advanced solid tumors"
Investors include OrbiMed, Vivo, Surveyor, Boxer, Pontifax, Samsara, Commodore, Janus & Aspire.
PR: mereobiopharma.com/news-and-event…
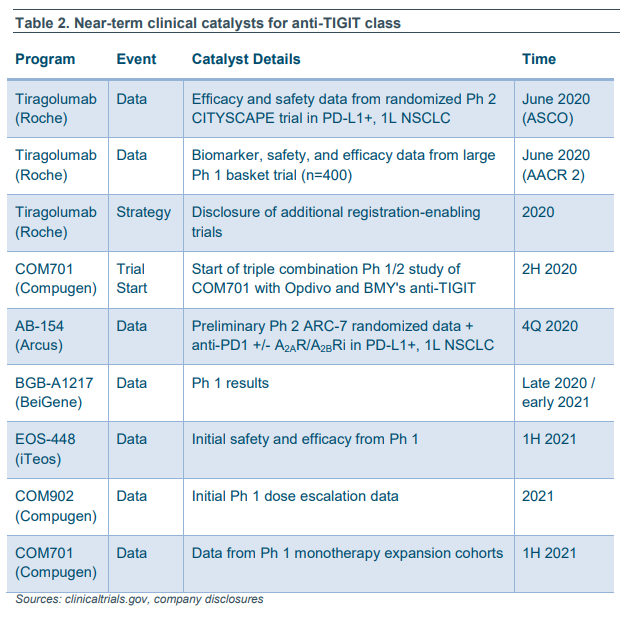
Next update for the TIGIT players is the release of Roche's #AACR20 abstract on 6/22/20.
investegate.co.uk/mereo-biopharm…
Mereo has sufficient drug product and plans to run a 60 patient Phase 1b study in combo with a PD-1 in selected tumor types - study to start in 4Q'20.
h/t @BertrandBio
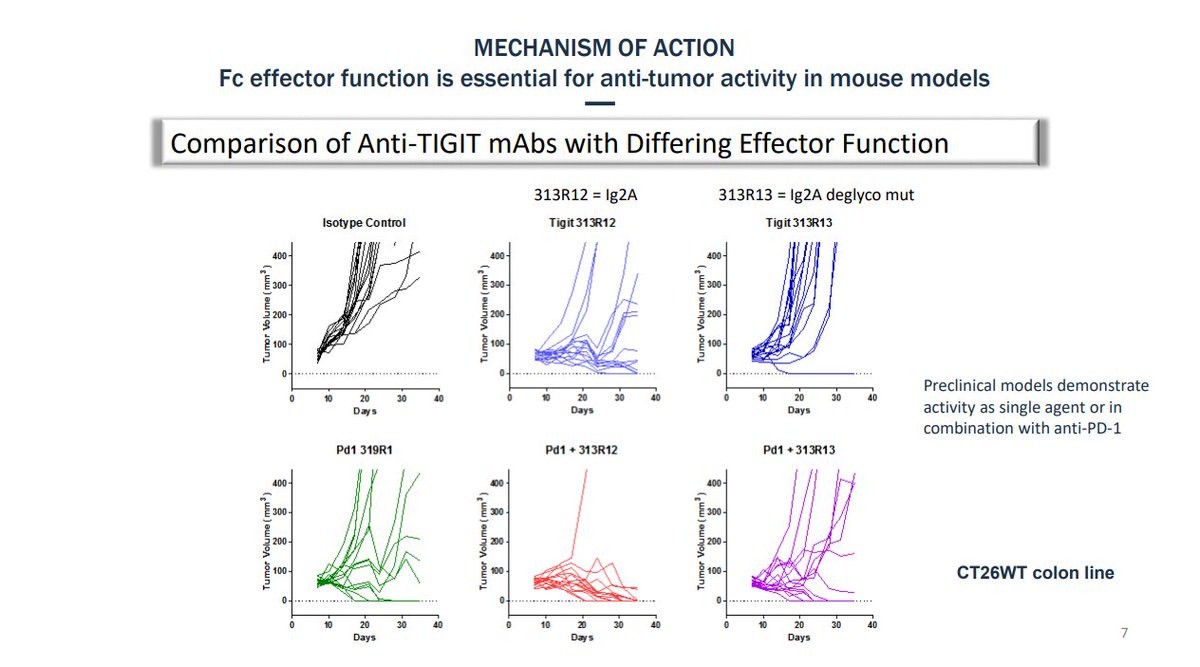
SEA-TGT is a nonfucosylated human IgG1 antibody.
Design: clinicaltrials.gov/ct2/show/NCT04…
- P1a monotherapy: 0% ORR (0/24)
- P1b Tecentriq combo: 3 responses in PD-L1+ tumors (NSCLC: 1 CR / 1 PR; HNSCC: 1 PR)
- #NSCLC expansion cohort with combo: 50% ORR (7/14) (1 CR / 6 PRs)
Update is at 9:40am ET.