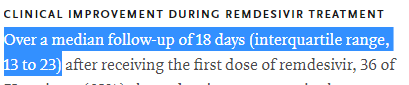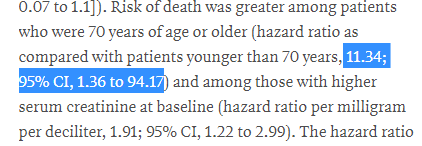A quick skim says to me that the paper is...bad
Let's do a live twitter critical appraisal and see! 1/n
That's a pretty big conflict of interest
It seems the paper was ghostwritten by a Gilead employee (who is NOT an author)
That's an amazing thing to have published in a @NEJM paper!!!

It also appears to go against @NEJM editorial guidelines which...isn't great?

Weird
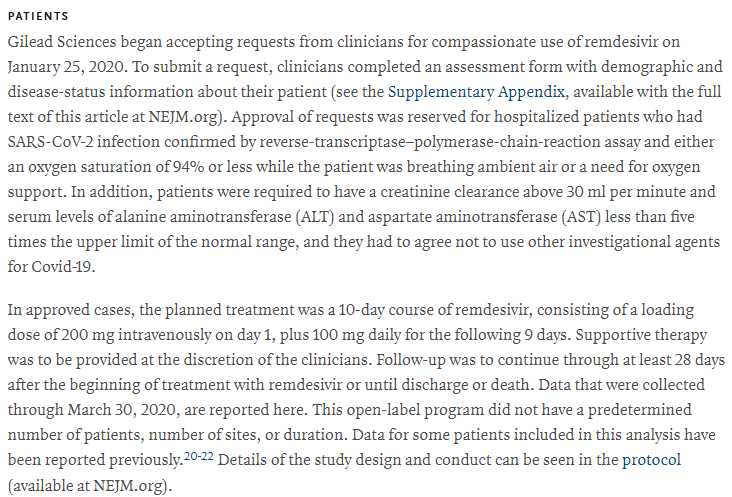
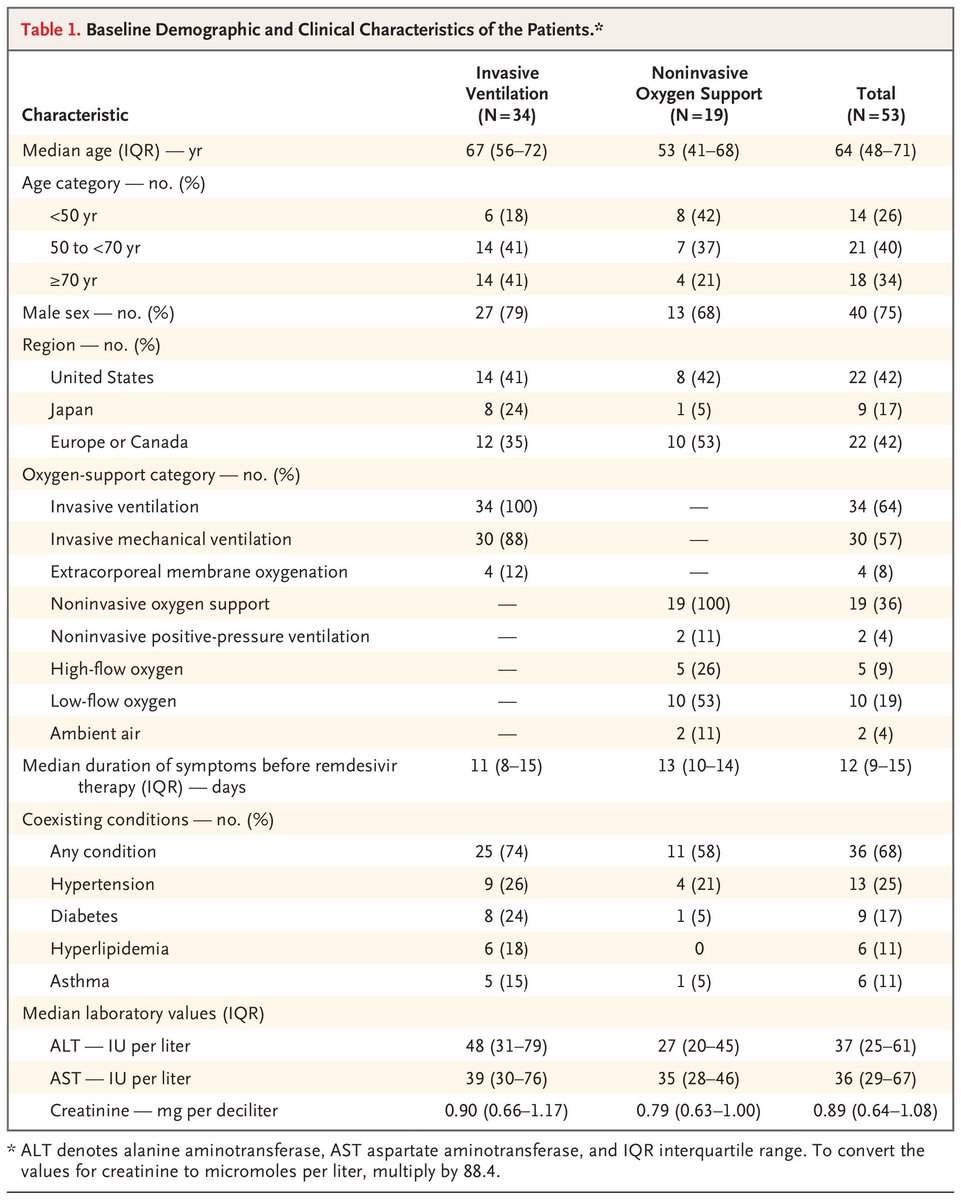
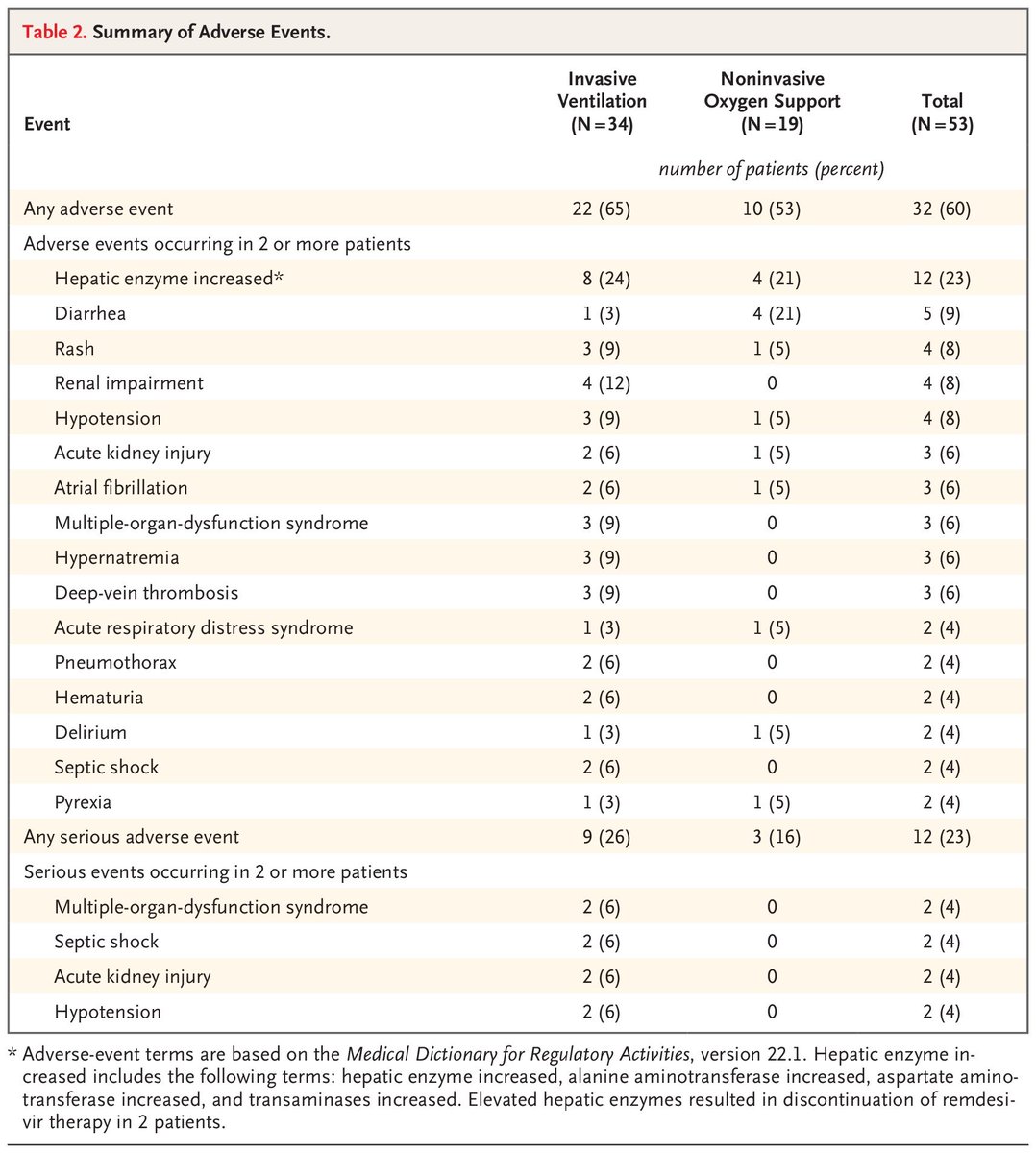
Of 61 total patients, 8 were excluded because of missing information
Of the remaining 53, only 40 received the full course of the drug
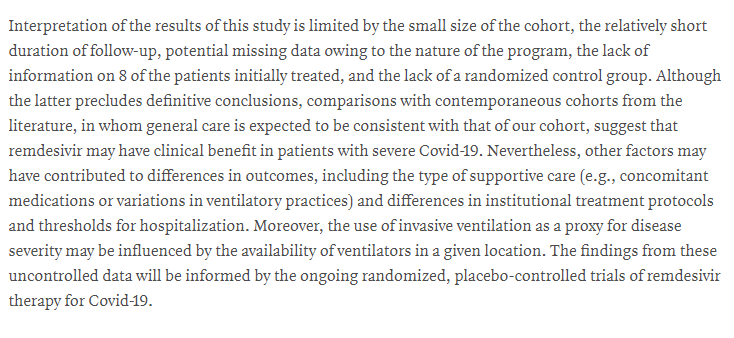
That's a huge problem for inference - how do we know if any improvement seen in this trial had anything to do with the drug?
It is extremely difficult to compare patients across trials, and absolutely NOT best practice
We also saw a high dropout rate in the trial, with 20% of patients not receiving the complete treatment!
Also, the patients were selected by their doctors - perhaps picking the patients that they thought had a fighting chance?
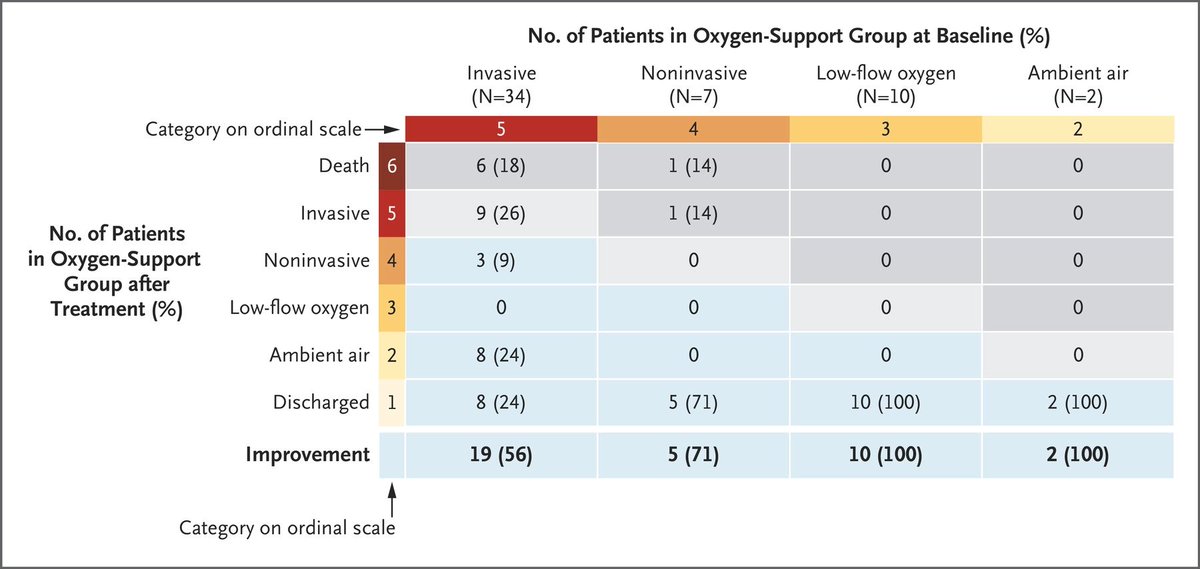
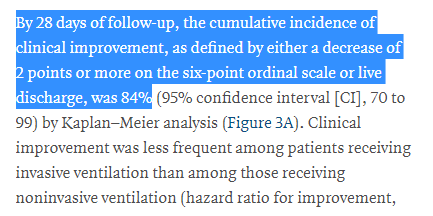
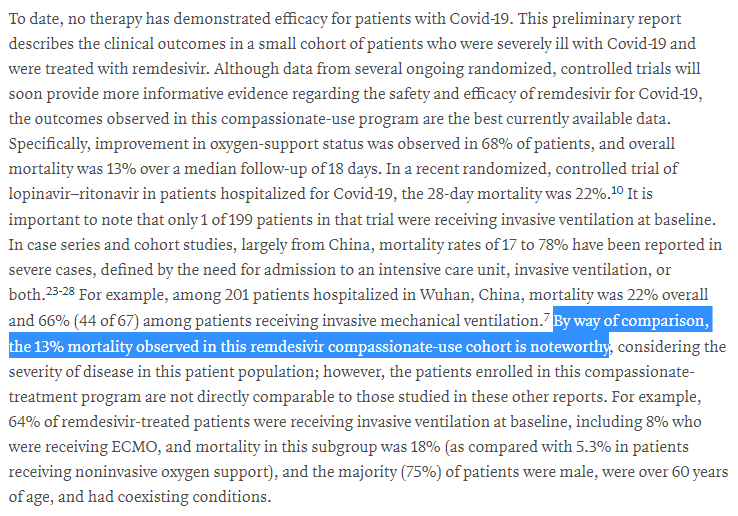
We can't really say whether the death rate was low or high from the data we have!
❌very small retrospective trial
❌no control group
❌written by pharma funder
❌high dropout
❌short timeframe
❌missing data
❌poorly written/edited
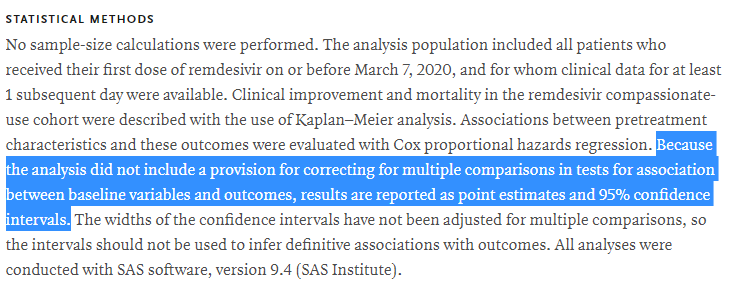
❌somewhat odd stats
❌highly selected patient cohort
✅no causal conclusions!
Hard to say anything more than that without a proper trial of some kind
I guess that's a question for @NEJM, who appear to have garnered millions of reads on the article in the last few days