Another study on #Hydroxycloroquine efficacy in #COVID19 patients from a Brazilian team has been shared widely this evening. Unfortunately I have to comment it because well it might have some public health implication & this study is atrocious
dropbox.com/s/5qm58cd4fnee…
dropbox.com/s/5qm58cd4fnee…
This study is not a clinical trial but a cohort study on 721 patients, recruited by telemedicine. Incl criteria Flu symptoms, OK to be treated > 18 y/o. Probable diagnosis of #SARSCoV2 but RT-PCR or X ray not compulsory. We don't even know if those patients had #COVID19 😳 😡🤦♂️
Outcome -> hospitalisation at day 7, that's it.
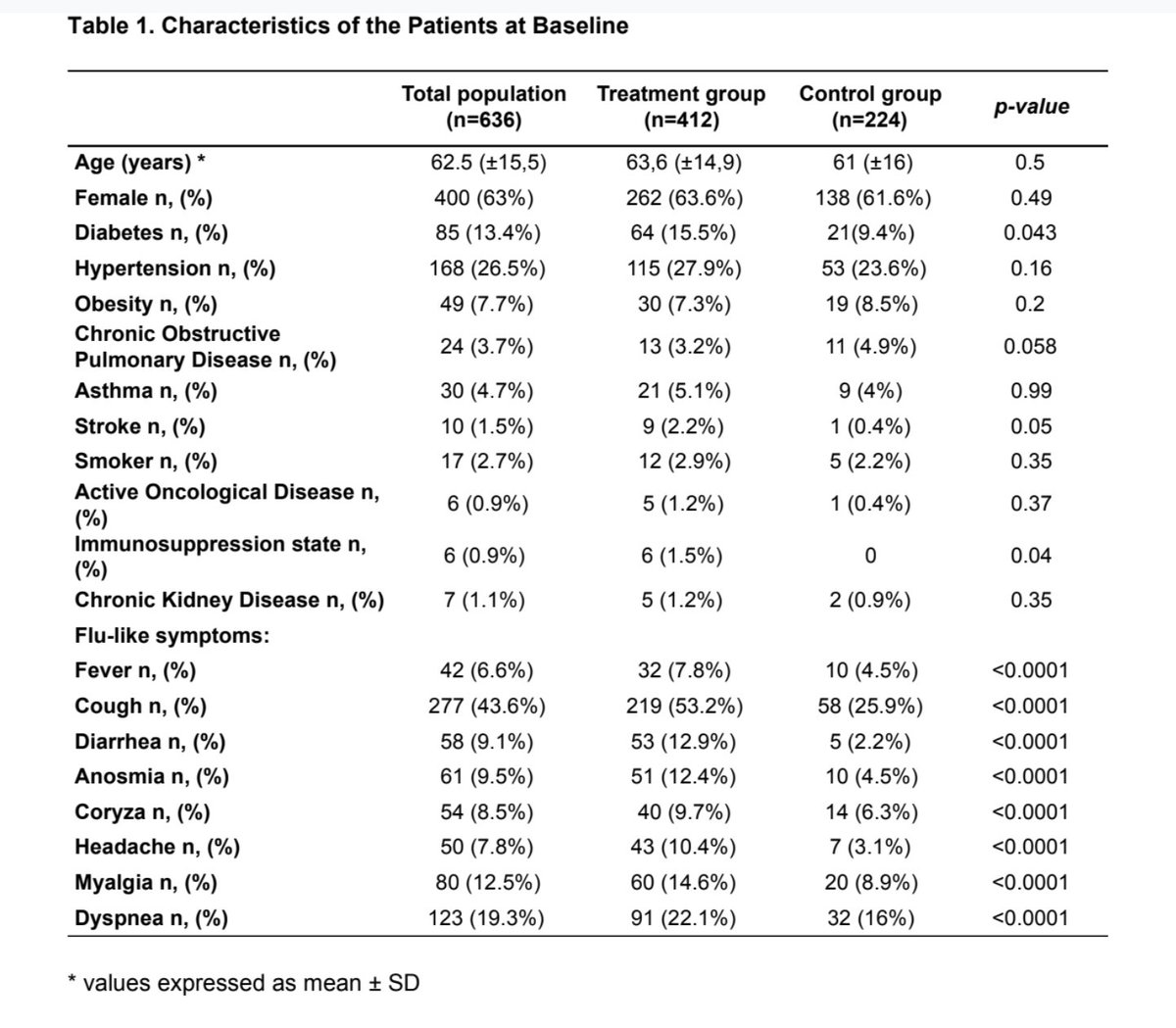
721 patients unrolled. 85 not followed -> 636 left => 225 refused treatment -> control group 😳 & 412 patients Hydroxycholoquine + azithromycine (dose unknown)
All followed daily by telemedicine consultation => huge select biais
721 patients unrolled. 85 not followed -> 636 left => 225 refused treatment -> control group 😳 & 412 patients Hydroxycholoquine + azithromycine (dose unknown)
All followed daily by telemedicine consultation => huge select biais
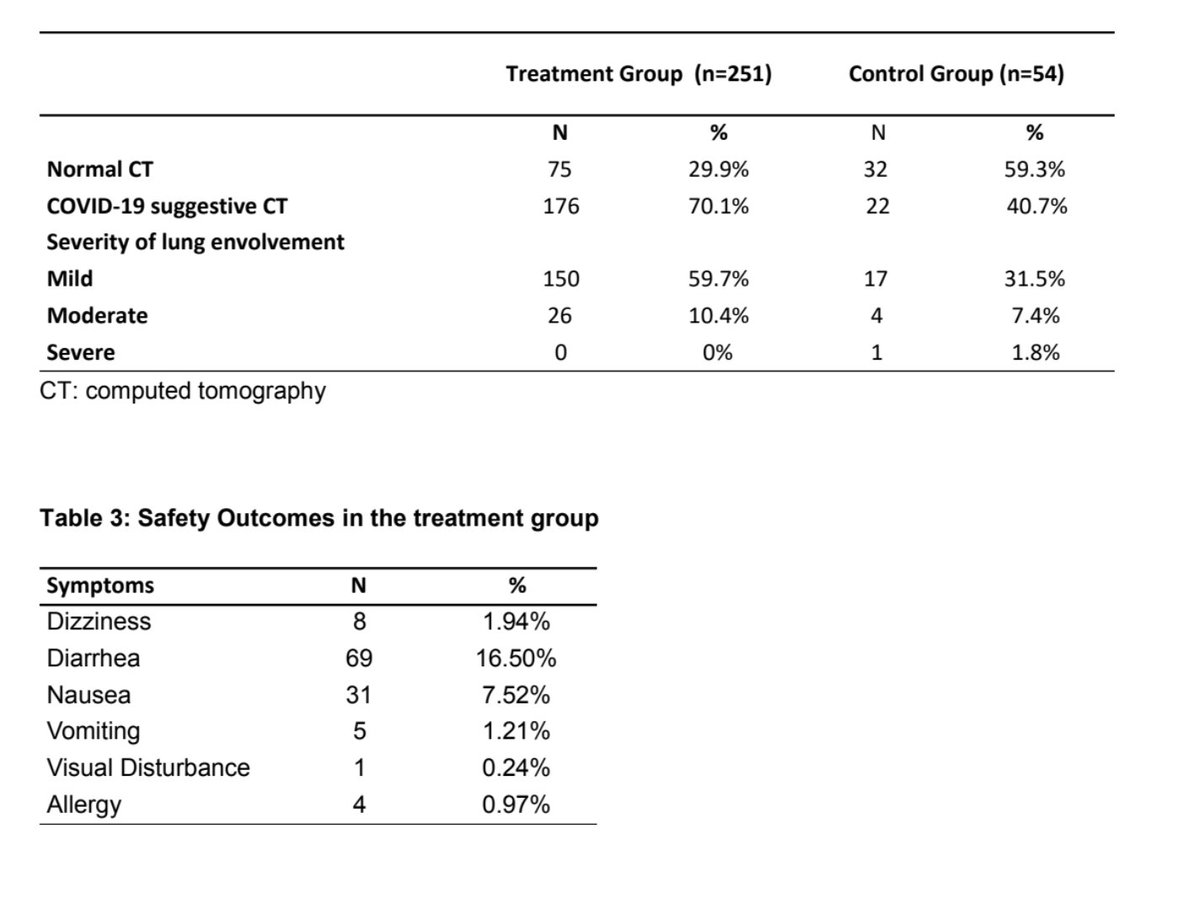
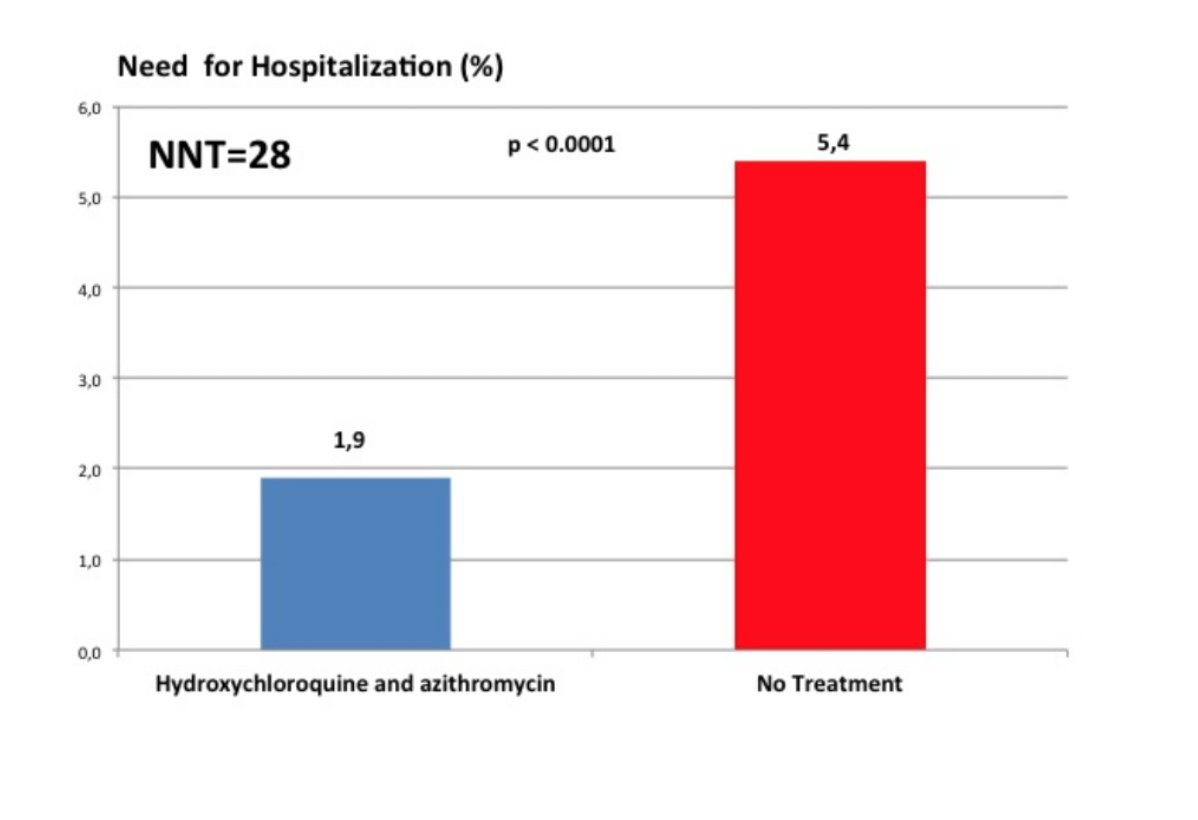
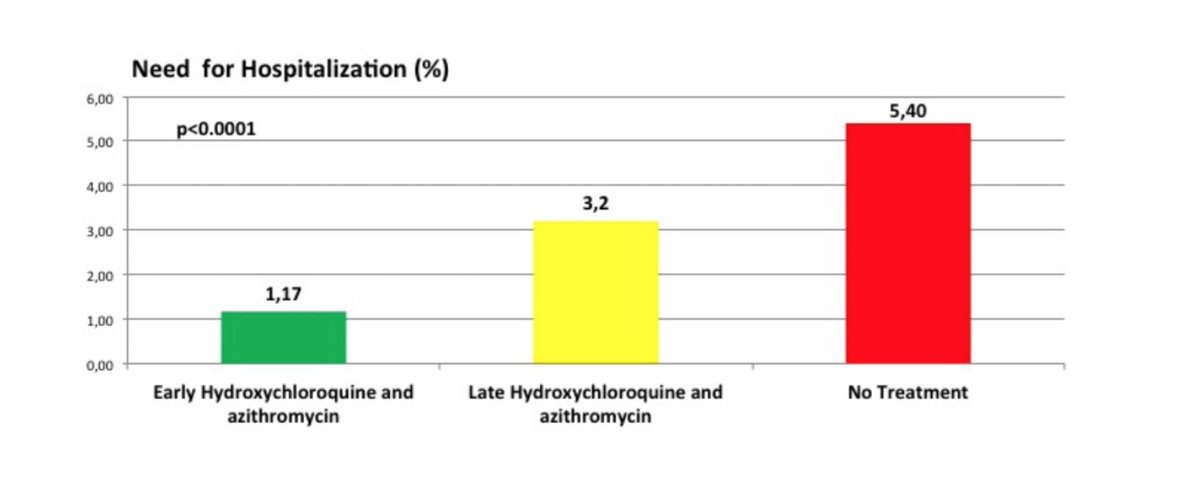
Outcome: HCQ+AZ group 1.9% hospitalisation versus 5.4% -> Chest CT scan for those hospitalized pattern compatible with #COVID19 infection but I don't know what that means given #SARS_CoV2 infection was not confirmed by RT-PCR 


In short from this study
1/ We don't know whether these patients were #SARSCoV2 diagnosed
2/ Huge selection bias, cases omitted % not blinded, not randomized and open label
3/ The results are meaningless. I don't know what this means
4/ The study is in short atrocious
😳 😡🤦♂️
1/ We don't know whether these patients were #SARSCoV2 diagnosed
2/ Huge selection bias, cases omitted % not blinded, not randomized and open label
3/ The results are meaningless. I don't know what this means
4/ The study is in short atrocious
😳 😡🤦♂️
Now there are others issues with this study.
1/ Clinical trial was registered 2 days ago and they are not yet recruiting 😳clinicaltrials.gov/ct2/show/NCT04…
1/ Clinical trial was registered 2 days ago and they are not yet recruiting 😳clinicaltrials.gov/ct2/show/NCT04…
2/ The study is performed from an insurance company in Brazil which has promoted its telemedicine application for #COVID19 ! So they have an interest to show efficacy of telemedicine against COVID19. the fact they have declared no COI is a joke
preventsenior.com.br/detalhes_notic…
preventsenior.com.br/detalhes_notic…

In conclusion, this is really really bad study and awful science, who knows that might have treated flu after all. Ethically it is very wrong. It is really sad. 😳 😡🤦♂️
• • •
Missing some Tweet in this thread? You can try to
force a refresh







