Recent negative results from $GILD and now $GNFT in Phase 3 highlight the uncertainties entering trials that patients face
Is there a minimum potential benefit a patient should accept when entering a trial?
THREAD
Is there a minimum threshold for benefit in a trial that participants might expect?
2/n
The majority thought that at least a 25% absolute improvement in either NASH or fibrosis was the minimum
3/n
We can focus on elafibranor since it provides a clear translational path from preclinical studies to early phase trials and then into phase 3
4/n
aasldpubs.onlinelibrary.wiley.com/doi/epdf/10.10…
This was enough to move into patients and the GOLDEN-505 trial
5/n
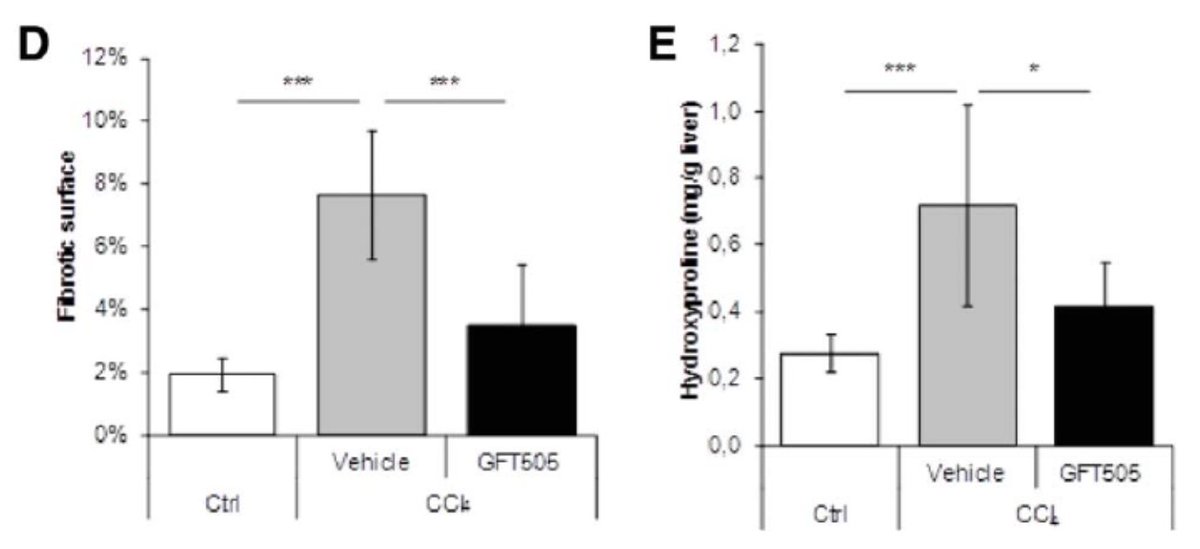
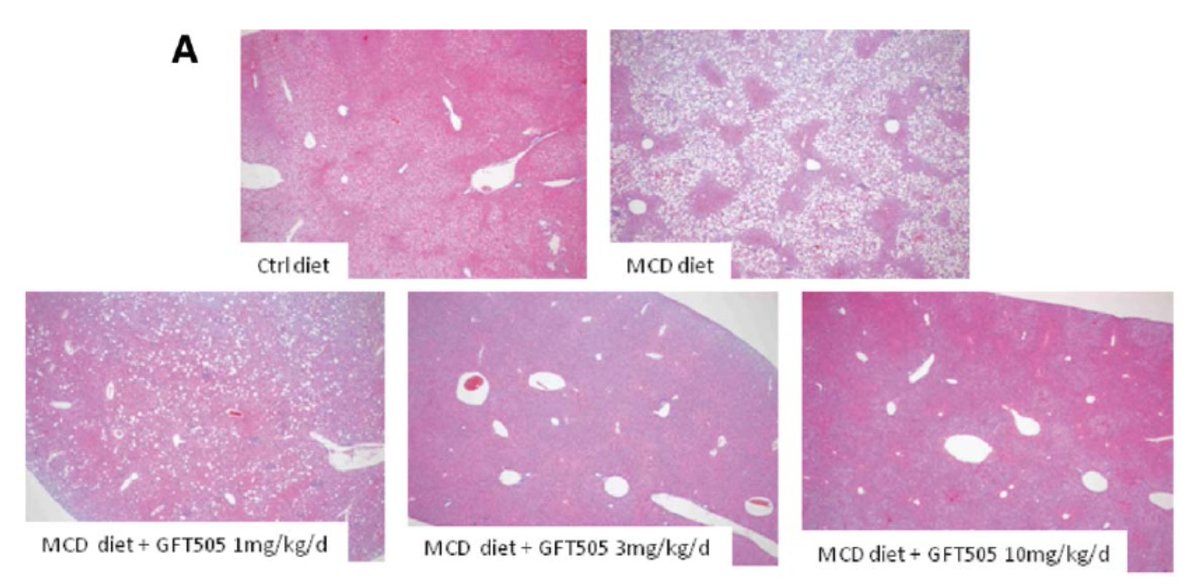
The endpoints in trials at this time were evolving and the authors presented the original and revised endpoints, focusing on a subgroup of patients with the most active NASH
gastrojournal.org/article/S0016-…
6/n
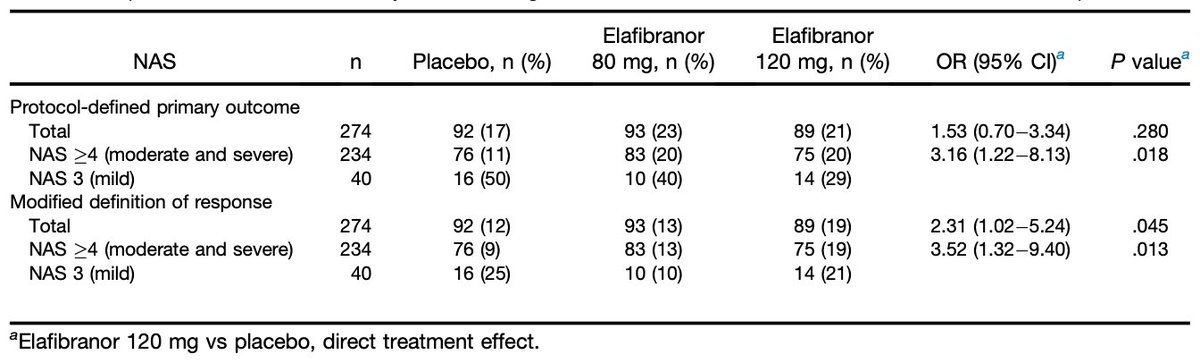
7/n
For instance, in the landmark piece from John Ioannidis, "Why most published research findings are false", the PPV for a true effect is <50%
doi.org/10.1371/journa…
8/n
ir.genfit.com/news-releases/…
9/n
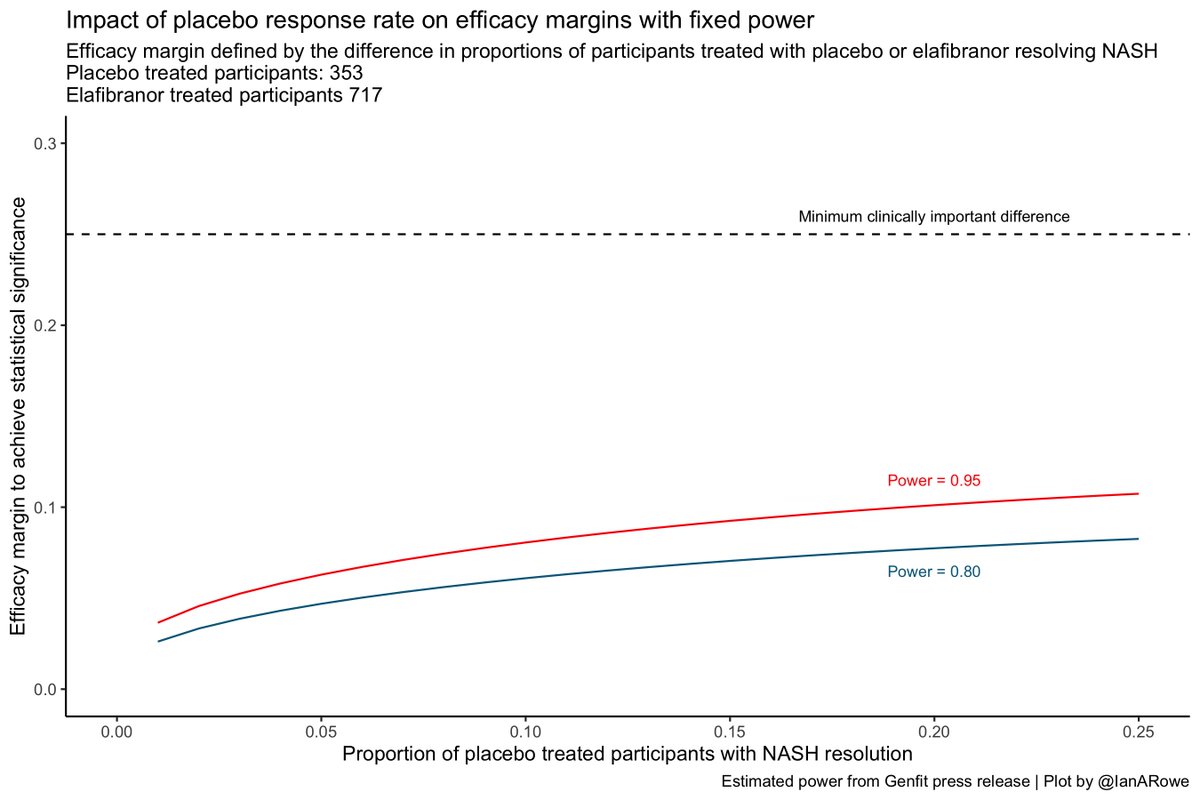
The press release gives us some insights into the study design, and importantly the efficacy margin that was planned for
Without seeing the protocol and power calculation we can only estimate, but the margin is narrow
10/n
Whilst the efficacy margin does increase with the placebo response to maintain power, this doesn't explain the lack of effect observed in the trial
12/n
1. Efficacy margins in phase 3 deserve attention
2. Placebo response rates don't have a major impact on efficacy margins
3. Animal models don't necessarily predict clinical efficacy
13/n
It will depend on the severity of disease and what alternatives are available
14/n
Histologic surrogates from phase 2 are hard to interpret but with time this will become clearer as clinical events occur in ongoing studies
15/n
How might it differ for patients with and without cirrhosis?
How can it best be measured?
ENDS