Why does alpha-1 antitrypsin deficiency (A1AT) cause liver disease (e.g. cirrhosis or hepatocellular carcinoma)?
I always assumed the mechanism was the same as for lung injury and emphysema, but it's not.
#tweetorial #medtwitter
Let's review the pathophys of A1AT:
- Alpha-1 antitrypsin (AAT) is a protease inhibitor, made in the liver, targeting neutrophil elastase
- Mutated alleles lead to decreased production of AAT (MM = normal genotype, ZZ = most common variant in A1AT)
pubmed.ncbi.nlm.nih.gov/29070580/
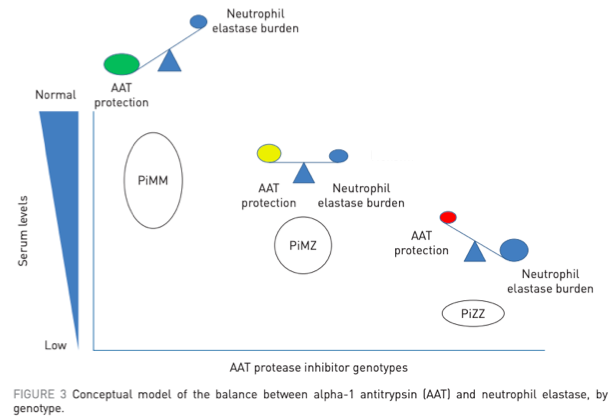
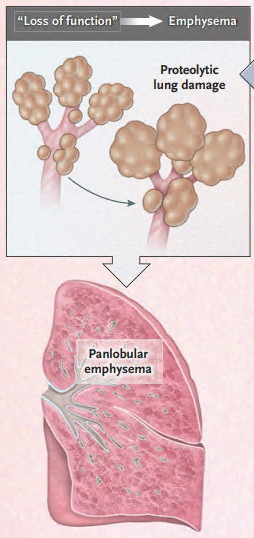
A1AT is most well known as an inherited cause of emphysema.
This occurs b/c of a "toxic loss of function" where elastase would normally be inhibited by AAT.
Loss of AAT production leads to uninhibited elastase activity and alveolar destruction.
pubmed.ncbi.nlm.nih.gov/32268028/
What about the mechanism of liver disease in A1AT?
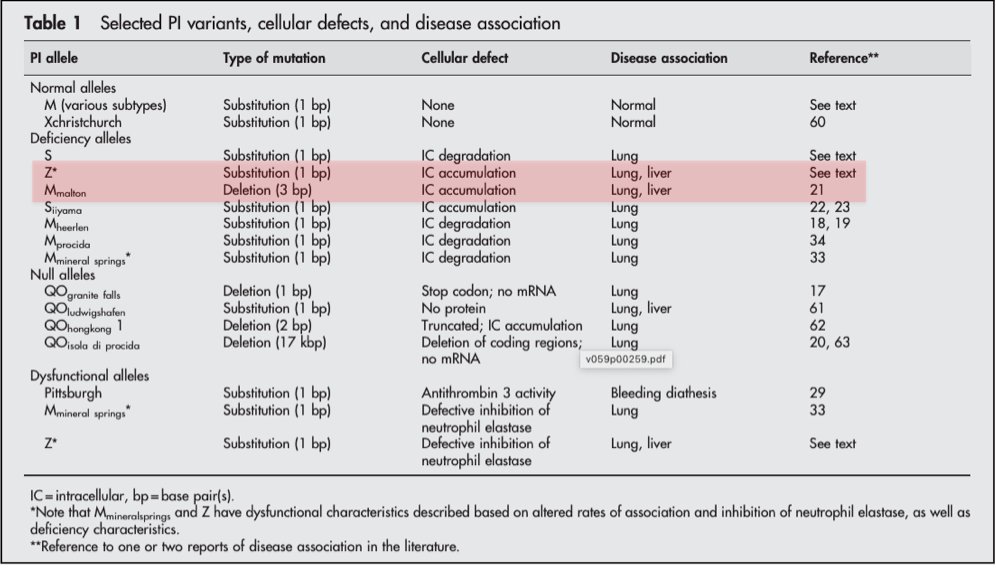
One clue is that only some AAT mutations lead to liver disease: Z (genotype ZZ) + M-malton (and, rarely, S).
Z is the most common mutation in A1AT and has the strongest association w/ liver disease.
pubmed.ncbi.nlm.nih.gov/14985567/
Next, let's learn the effects of the Z mutation to see why it can cause liver disease.
With Z, lysine substitutes for glutamic acid at a hinge point in AAT.
This causes protein misfolding/polymerization in the hepatocyte endoplasmic reticulum (ER).
pubmed.ncbi.nlm.nih.gov/1608473/
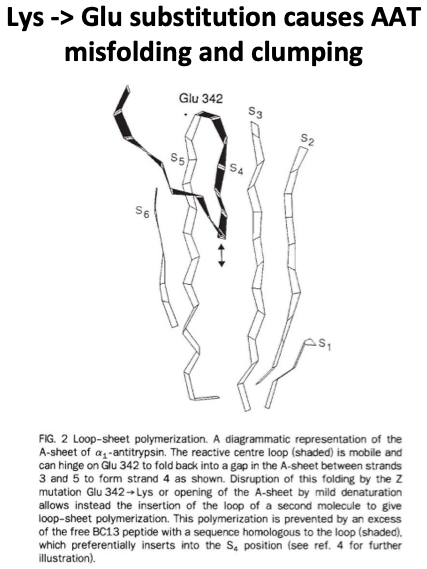
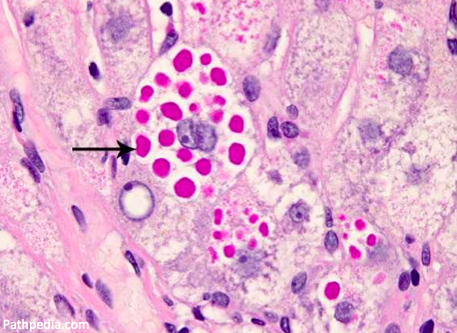
Polymerized AAT clumps together in hepatocytes, leading to histologically visible, PAS-positive inclusions.
⚡️This accumulation is called a "toxic gain of function", as opposed to the toxic loss of function that leads to emphysema.
pubmed.ncbi.nlm.nih.gov/28752441/
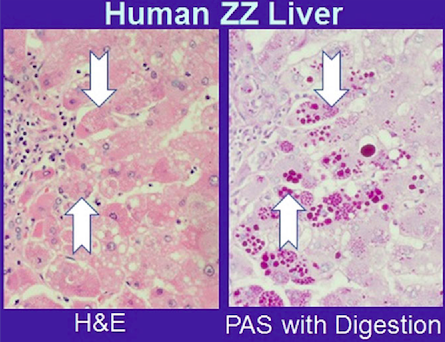
A follow-up question: how do these clumps of AATs actually lead to hepatic injury?
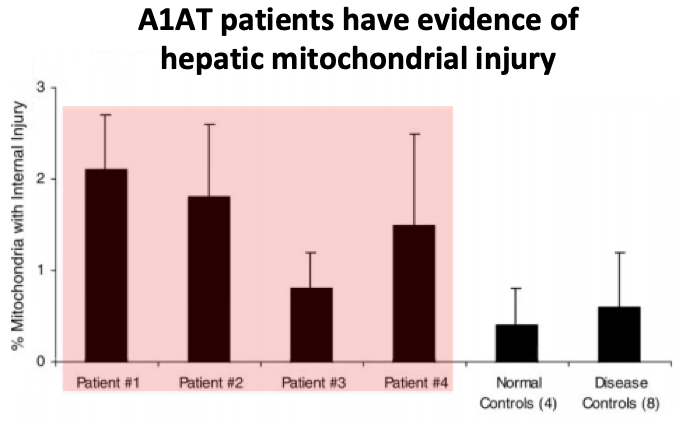
The exact causes are not known but the leading theory involves mitochondrial damage...
Mouse A1AT models and human patient studies have shown that clumps of AAT in the ER injure hepatic mitochondria, in a process called proteotoxic stress.
The injured mitochondria release reactive oxygen species, contributing to hepatocyte damage.
pubmed.ncbi.nlm.nih.gov/14684378/
A "null" genotype, where no circulating AAT is produced at all, get severe emphysema (b/c they make no AAT). But liver disease has never been observed.
This reinforces that the key event for liver disease development is hepatocyte AAT accumulation.
pubmed.ncbi.nlm.nih.gov/2254451/
The mechanism of liver disease in A1AT has therapeutic implications as well.
For patients with severe deficiencies of AAT and emphysema, pooled donor plasma w/ AAT is often used therapeutically to replace the enzyme they don't produce.
pubmed.ncbi.nlm.nih.gov/22536580/
💥 But because liver disease results from intra-hepatocyte inclusions of AAT, not protease deficiency, enzyme replacement has no role.
Let's end w/ an interesting association:
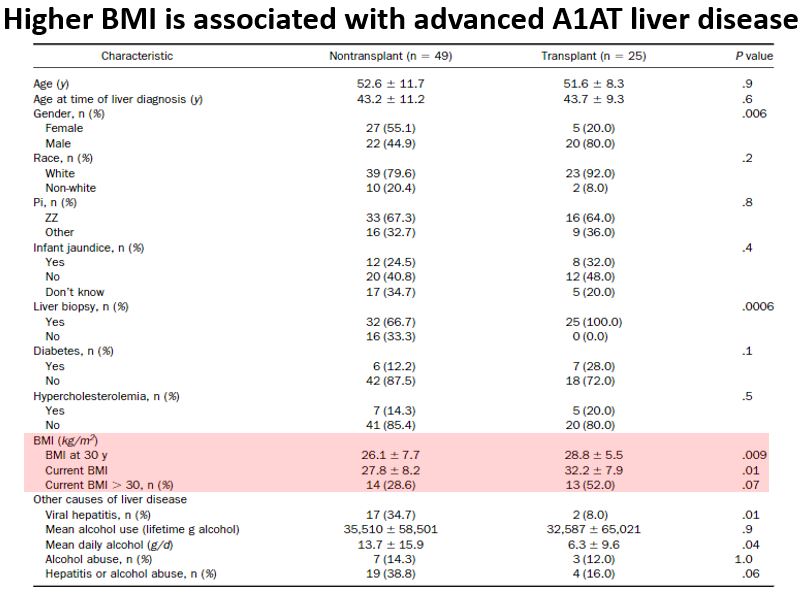
A1AT liver disease and obesity appear to be linked.
For one, obesity is a primary risk factor for progression of A1AT liver disease to cirrhosis.
pubmed.ncbi.nlm.nih.gov/15822045/
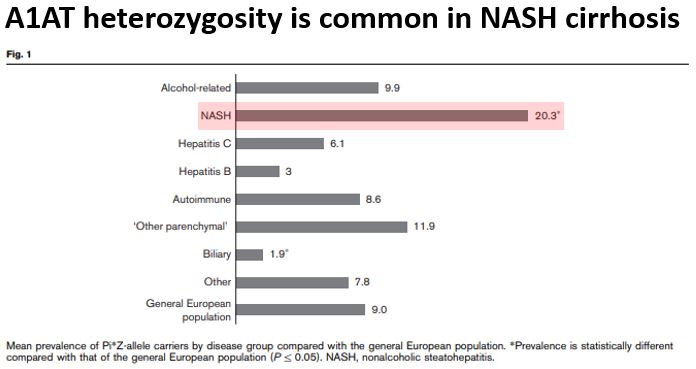
And, amazingly, heterozygosity for A1AT alleles is associated w/ progression of fatty liver disease to NASH cirrhosis.
In one study, 20% of patients w/ NASH cirrhosis had one Z mutation (much higher than would be expected in the general population).
pubmed.ncbi.nlm.nih.gov/31597010/
Why would heterozygosity for the Z AAT allele worsen NASH?
Z heterozygotes do make some abnormal AAT. It may be that inflammatory injury from NASH interferes w/ removal of abnormal AAT (autophagy), exacerbating the underlying fatty liver disease.
pubmed.ncbi.nlm.nih.gov/31597010/
💡A1AT liver dx is due to AAT misfolding and clumping in hepatocytes, injuring mitochondria
💡= "toxic gain of function"
💡Differs from "toxic loss of function" in A1AT emphysema
💡Link b/w A1AT and obesity (obesity ⬆️ A1AT liver dx, Z allele ⬆️ risk for NASH cirrhosis)