Ever wonder why metronidazole (Flagyl) treats both bacteria and parasites?
The answer may also explain the infamous disulfiram-like reaction that can occur when metronidazole is mixed with alcohol.
#tweetorial #medtwitter
First let's review metronidazole's spectrum of activity
It is bacteriocidal against:
- Anaerobic gram negative/gram positive bacteria
- Microaerophilics like H. pylori
It's also active against anaerobic protozoa like giardia or entamoeba histolytica
ncbi.nlm.nih.gov/pmc/articles/P…
Metronidazole was developed in the 1950s to treat trichomonas, and found to be active against giardia and entamoeba. Only later was it tested for anaerobic bacteria.
I always thought of it as an antibiotic that treats parasites. The opposite is true.
ncbi.nlm.nih.gov/pubmed/151090
So how does metronidazole kill anaerobic bacteria and protozoa?
To understand this we need to review pyruvate metabolism.
It turns out that aerobic organisms (like us) metabolize pyruvate differently from anaerobic organisms.
Aerobes use the pyruvate dehydrogenase complex (PDC) to convert pyruvate to acetyl-CoA, which then feeds into the Krebs (aka citrate) Cycle, generating ATP.
ncbi.nlm.nih.gov/pubmed/30001904
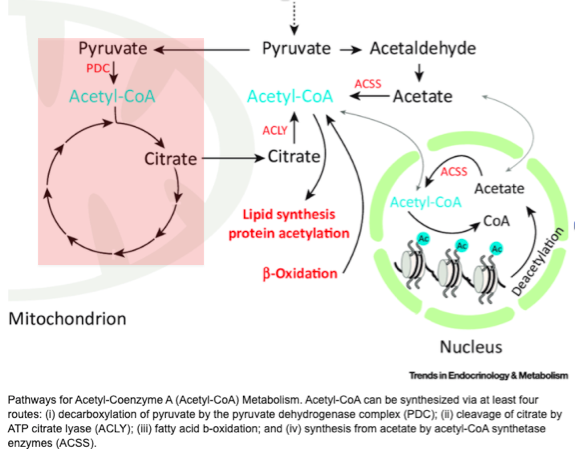
Anaerobic bacteria and parasites metabolize pyruvate differently.
They do convert pyruvate to acetyl-CoA, but use an enzyme called pyruvate:ferredoxin oxidoreductase (PFOR).
This red-ox reaction generates electrons that are transferred to NADP+.
jb.asm.org/content/189/13…
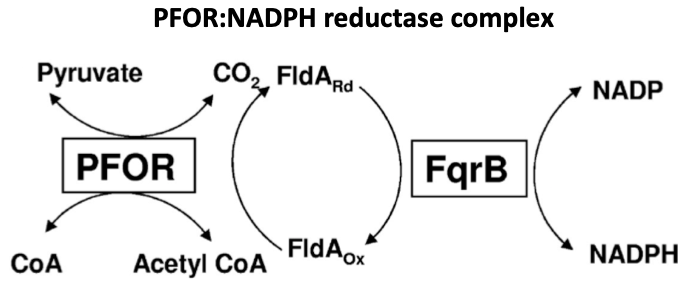
What does this have to do w/ metronidazole?
Upon entering anaerobes, metronidazole (instead of NADP+) is reduced by PFOR.
Now activated, it accumulates electrons ➡️ forms superoxide anions ➡️ damages DNA ➡️ kills the organism (bacteria or parasite).
ncbi.nlm.nih.gov/pmc/articles/P…
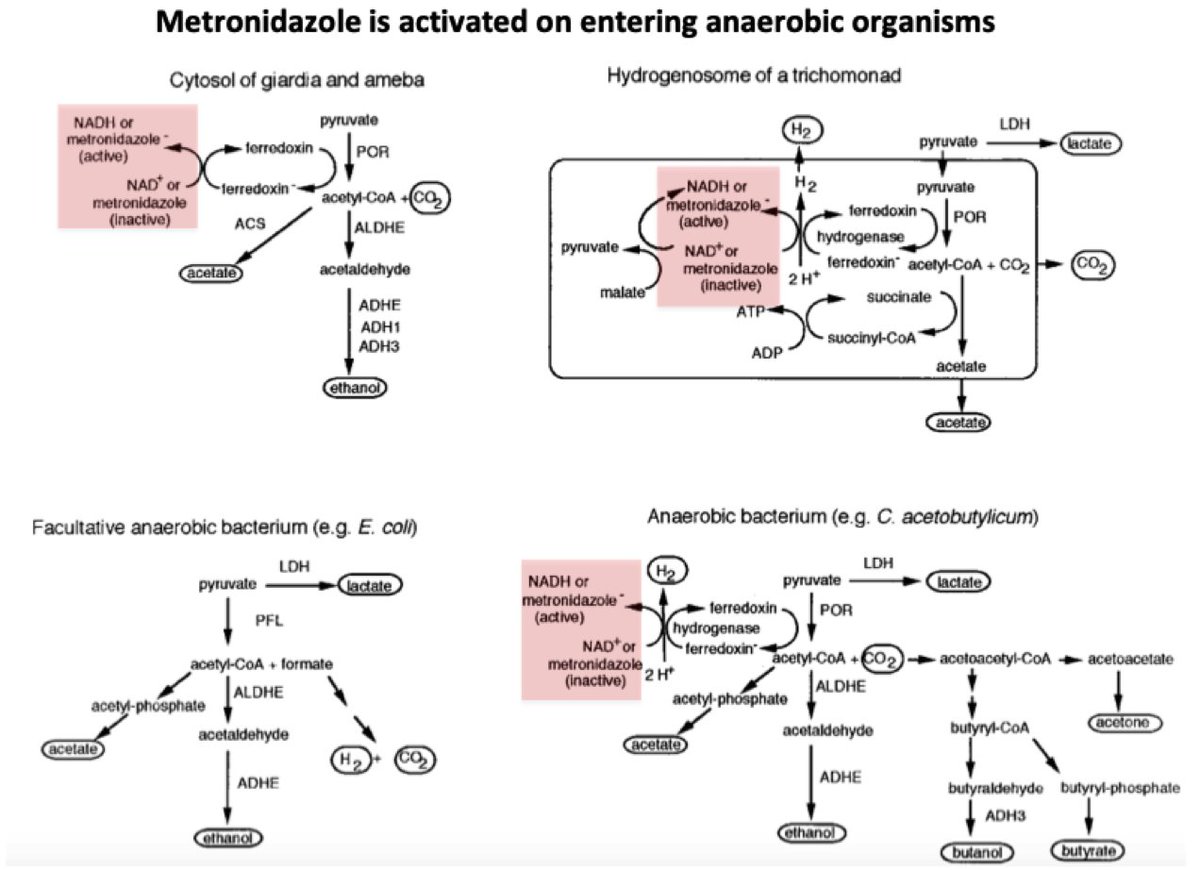
Based on this mechanism of action (DNA damage), you might expect that metronidazole would be severely toxic to human cells.
⚡️ But because it requires activation by specifically anaerobic machinery, it doesn't damage our own DNA.
It's actually an incredibly elegant drug.
A cool correlate:
Metronidazole can cause a disulfiram-like reaction if mixed w/ alcohol: flushing, nausea/vomiting, headache.
The actual disulfiram-alcohol reaction is caused by inhibition of hepatic aldehyde dehydrogenase and ⬆️ acetaldehyde.
researchgate.net/publication/28…
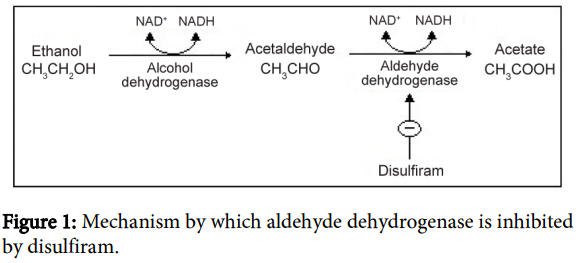
Metronidazole doesn't inhibit hepatic alcohol dehydrogenase like disulfiram does.
But one theory for the disulfiram-like reaction involves metronidazole's effect on colonic anaerobic bacteria...
Take a closer look at what happens to acetyl-coA after it's formed (figure in tweet #7). It's metabolized to acetaldehyde and then ethanol.
Aerobic bacteria have the ability to run this reaction in reverse, forming acetaldehyde from ingested alcohol.
ncbi.nlm.nih.gov/pubmed/9161610
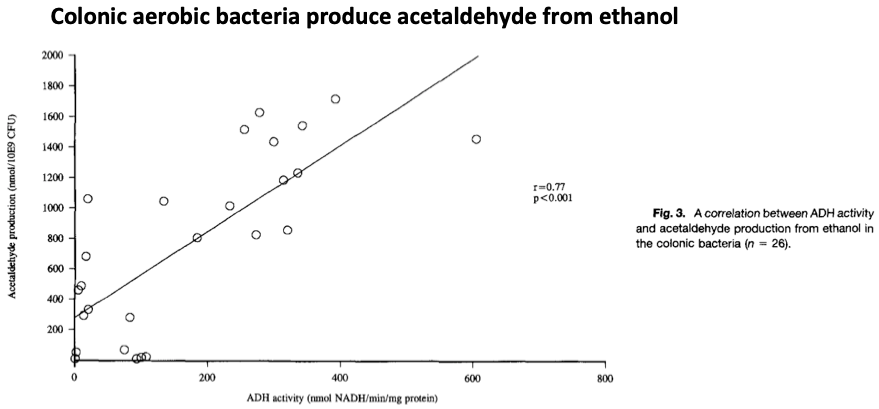
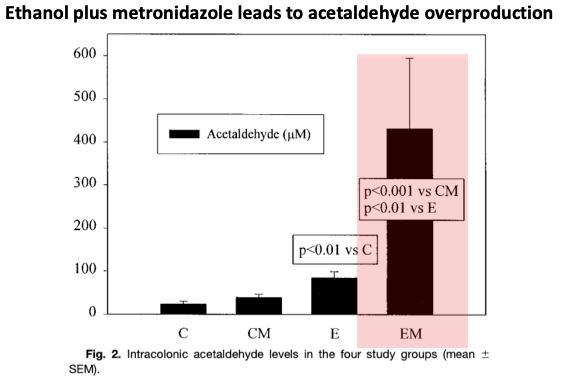
Because metronidazole only kills anaerobes, aerobic bacteria in the colon can overproduce acetaldehyde in response to alcohol ingestion, as was found in this experiment in rats.
This increase in acetaldehyde may lead to the disulfiram-like reaction.
ncbi.nlm.nih.gov/pubmed/10798595
@tony_breu just published a #tweetorial that touched on some of the effects of acetaldehyde, if you want to learn more 👇
💡 Pyruvate:ferredoxin oxidoreductase (PFOR) activates metronidazole
💡 PFOR is unique to anaerobic bacteria/parasites
💡 After activation, superoxide radicals form and damage DNA, selectively killing anaerobes
💡 The disulfiram-like reaction may be caused by this selectivity