#MXCT
MaxCyte is a cell engineering platform, which uses non viral mRNA for drug development in a wide range of diseases including
-Cancer
-Auto Immune Diseases
11 Commercial Licencing deals with over
-$800m in Milestone Payments
MaxCyte is a cell engineering platform, which uses non viral mRNA for drug development in a wide range of diseases including
-Cancer
-Auto Immune Diseases
11 Commercial Licencing deals with over
-$800m in Milestone Payments

#MXCT
MaxCyte is based on their patented novel drug development approach which is Flow Electroporation
Flow Electroporation is reprogramming of Human T-Cells
What this means is they have a machine that can modify any human/patients cells in order to target a specific problem
MaxCyte is based on their patented novel drug development approach which is Flow Electroporation
Flow Electroporation is reprogramming of Human T-Cells
What this means is they have a machine that can modify any human/patients cells in order to target a specific problem

#MXCT
This as I hope you will understand offers enormous potential to address a wide range of therapeutic issues all under the platform which they own
This as I hope you will understand offers enormous potential to address a wide range of therapeutic issues all under the platform which they own
#MXCT
Some of the partners listed below exploring cell therapy are world leaders in the field.
Crispr partners also with CureVac (a mRNA company) which is backed by the BMGF, highlighting the area of work is becoming an increasing sought after therapeutic division

Some of the partners listed below exploring cell therapy are world leaders in the field.
Crispr partners also with CureVac (a mRNA company) which is backed by the BMGF, highlighting the area of work is becoming an increasing sought after therapeutic division


#MXCT
Now what gets me excited is their approach to Cancer, they have recently set up CARMA subsidiary
CARMA is the use of CAR T-Cells in Solid Tumour Cancers, CAR T-Cells have never been proven to work in solid tumours but MXCT are showing early positive indications
Now what gets me excited is their approach to Cancer, they have recently set up CARMA subsidiary
CARMA is the use of CAR T-Cells in Solid Tumour Cancers, CAR T-Cells have never been proven to work in solid tumours but MXCT are showing early positive indications

#MXCT
MaxCyte have set up a US Boutique to manage the financing and listing on a main US Exchange which is anticipated in 2021
(Post Phase 1 Data announcement for their study of MCY-M11 In Ovarian Cancer)
1 Specialist Life Science US Investor has already invested
MaxCyte have set up a US Boutique to manage the financing and listing on a main US Exchange which is anticipated in 2021
(Post Phase 1 Data announcement for their study of MCY-M11 In Ovarian Cancer)
1 Specialist Life Science US Investor has already invested
#MXCT
MCY-M11
Phase 1 is an open label dose escalation study for the treatment of Ovarian Cancer
So far they have seen mild side effects with initial encouraging efficacy in preventing disease progression in cohorts 2 and 3
MCY-M11
Phase 1 is an open label dose escalation study for the treatment of Ovarian Cancer
So far they have seen mild side effects with initial encouraging efficacy in preventing disease progression in cohorts 2 and 3

#MXCT
MCY-M11
Note
This Phase 1 Trial is to assess the safety profile of the drug at different concentrations before conducting a prolonged dose study at an optimal concentration
Stable disease has been achieved for 6 months in Reoccurring Ovarian Cancer after lines of Chemo
MCY-M11
Note
This Phase 1 Trial is to assess the safety profile of the drug at different concentrations before conducting a prolonged dose study at an optimal concentration
Stable disease has been achieved for 6 months in Reoccurring Ovarian Cancer after lines of Chemo
#MXCT
MCY-M11
Note this stable disease was achieved in Cohort 2, a low dose for a short duration (3 weeks) yielding stable control of the cancer
This has formed an encouraging early clinical base for both safety and potential efficacy with prolonged repeated cycles
MCY-M11
Note this stable disease was achieved in Cohort 2, a low dose for a short duration (3 weeks) yielding stable control of the cancer
This has formed an encouraging early clinical base for both safety and potential efficacy with prolonged repeated cycles
#MXCT
CARMA Platform
-This platform extracts an individuals own immune cells (T Cells)
-Genetically Modifies them to specifically target +kill cancer tumours
-Whilst doing this it also programmes the bodies immune system to self manufacture the genetically modified immune cells
CARMA Platform
-This platform extracts an individuals own immune cells (T Cells)
-Genetically Modifies them to specifically target +kill cancer tumours
-Whilst doing this it also programmes the bodies immune system to self manufacture the genetically modified immune cells

#MXCT
This is the quickest CAR T Cell development globally.
With the ability to manufacture these genetically modified personalised cancer therapy within 1 day compared to 2-6 weeks for similar CAR T-Cell therapies
MCY-M11 -Phase 1 Ovarian Cancer Read Out H2 2020
This is the quickest CAR T Cell development globally.
With the ability to manufacture these genetically modified personalised cancer therapy within 1 day compared to 2-6 weeks for similar CAR T-Cell therapies
MCY-M11 -Phase 1 Ovarian Cancer Read Out H2 2020
#MXCT
MCY-M11
Ovarian Cancer is a highly unmet area within oncology with only a few approved drugs, the focus on trying to delay tumour progression is the initial primary objective meaning the scope for improvement is huge
MCY-M11
Ovarian Cancer is a highly unmet area within oncology with only a few approved drugs, the focus on trying to delay tumour progression is the initial primary objective meaning the scope for improvement is huge

#MXCT
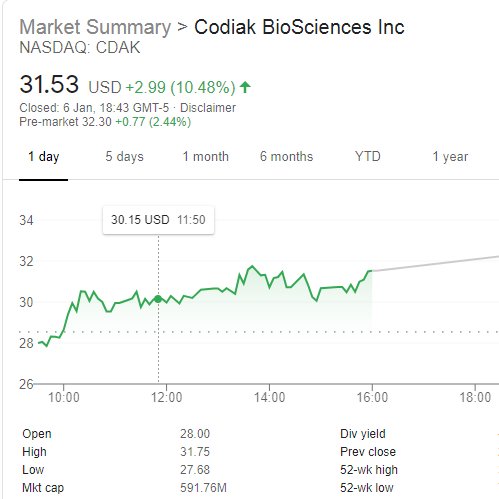
Classic Case of an AIM listed Pharma- 🚨MASSIVELY UNDERVALUED🚨 to its less advanced peer listed on the NASDAQ
That will change shortly as MXCT intends to list on NASDAQ Q1 2021
📈
Classic Case of an AIM listed Pharma- 🚨MASSIVELY UNDERVALUED🚨 to its less advanced peer listed on the NASDAQ
That will change shortly as MXCT intends to list on NASDAQ Q1 2021
📈
https://twitter.com/AimHardy/status/1290544133870956545?s=20
#MXCT
MaxCyte announce the development of their EXCITING
-mRNA CAR-T Cell Therapy for Cancer
which will list on NASDAQ in Q1 2021 under their CARMA Subsidiary
The announcement entails the next stage of demonstrating efficacy as I will now go on to explain
MaxCyte announce the development of their EXCITING
-mRNA CAR-T Cell Therapy for Cancer
which will list on NASDAQ in Q1 2021 under their CARMA Subsidiary
The announcement entails the next stage of demonstrating efficacy as I will now go on to explain

#MXCT
What is TREMENDOUS about this?
With only 1 single 3 week cycle, stable disease was achieved for 6 months💥
With the addition of Cyclophosphamide
-an immune pre conditioner
it is likely less side effects will occur and with repeat lower dose cycles administered it is...


What is TREMENDOUS about this?
With only 1 single 3 week cycle, stable disease was achieved for 6 months💥
With the addition of Cyclophosphamide
-an immune pre conditioner
it is likely less side effects will occur and with repeat lower dose cycles administered it is...



#MXCT
Highly likely stronger efficacy will be achieved. Showing Tumour regression and minimal side effects
CAR T Therapy is a very hot sector in oncology development right now, with this completely Novel Approach, MaxCyte is primed for a re rate over the following 12 months📈💥
Highly likely stronger efficacy will be achieved. Showing Tumour regression and minimal side effects
CAR T Therapy is a very hot sector in oncology development right now, with this completely Novel Approach, MaxCyte is primed for a re rate over the following 12 months📈💥
#MXCT🧬
Mr Calvin on @MaxCyte_info 's mRNA therapy
"It's exciting to think that the work we do at MaxCyte to design products to meet the needs of our customers helps to bring us closer to 💥breakthrough therapies💥 for patients.
Mr Calvin on @MaxCyte_info 's mRNA therapy
"It's exciting to think that the work we do at MaxCyte to design products to meet the needs of our customers helps to bring us closer to 💥breakthrough therapies💥 for patients.

#MXCT🧬
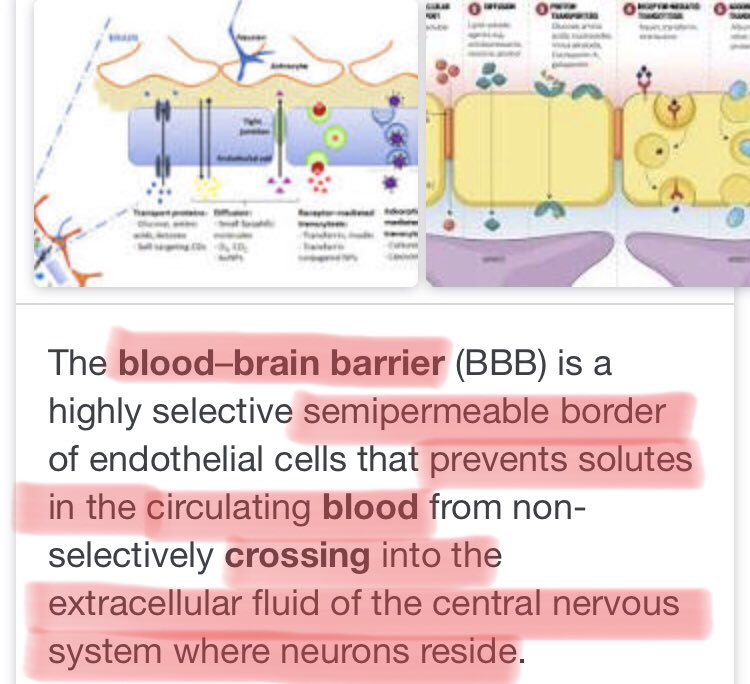
MaxCyte’s webinar on the ongoing relationship with CRISPR on finding a potential C U R E for Sickle Cell Disease
A disease which distorts Red Blood Cell Shape and ultimately leads to deficiencies, strokes and lung problems
MaxCyte’s webinar on the ongoing relationship with CRISPR on finding a potential C U R E for Sickle Cell Disease
A disease which distorts Red Blood Cell Shape and ultimately leads to deficiencies, strokes and lung problems
https://twitter.com/MaxCyte_info/status/1316740181412765698
#MXCT🧬
🔥Ground breaking🔥 pre clinical and mouse models suggest
This therapy
🚨CURES 30 - 50%🚨
of gene malfunction resulting in potentially normal function of Red Blood Cells
No Significant Toxicity events ✅
First patients to be dosed H1 2021 📈

🔥Ground breaking🔥 pre clinical and mouse models suggest
This therapy
🚨CURES 30 - 50%🚨
of gene malfunction resulting in potentially normal function of Red Blood Cells
No Significant Toxicity events ✅
First patients to be dosed H1 2021 📈


#MXCT 🧬
This article demonstrates how MaxCyte's technology can successfully Genetically Engineer Human Immune Cells to make them more powerful at recognising and killing Cancer Tumours💥
Something which Iovance and Fate Therapeutics are working on📈
maxcyte.com/wp-content/upl…
This article demonstrates how MaxCyte's technology can successfully Genetically Engineer Human Immune Cells to make them more powerful at recognising and killing Cancer Tumours💥
Something which Iovance and Fate Therapeutics are working on📈
maxcyte.com/wp-content/upl…
#MXCT 🧬
This article demonstrating superior transfection efficiency using MaxCytes technology including superiority over the widely adopted CRISPR spCAS9 $CRSP
maxcyte.com/wp-content/upl…
This article demonstrating superior transfection efficiency using MaxCytes technology including superiority over the widely adopted CRISPR spCAS9 $CRSP
maxcyte.com/wp-content/upl…
#MXCT 🧬
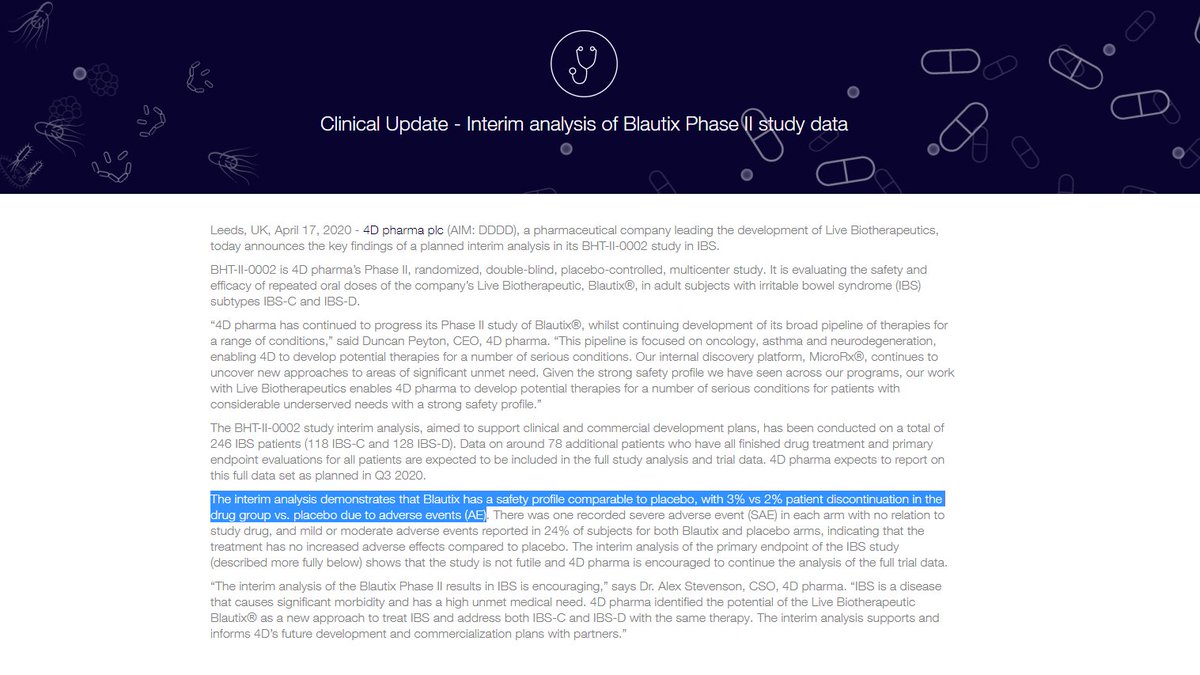
Important to note adoption of their innovative technology is gaining traction in one of the hottest sectors in the world right now (Cell and Gene Therapy)
As they report financial results are expected to be ahead of market expectations
Important to note adoption of their innovative technology is gaining traction in one of the hottest sectors in the world right now (Cell and Gene Therapy)
As they report financial results are expected to be ahead of market expectations

#MXCT 🧬
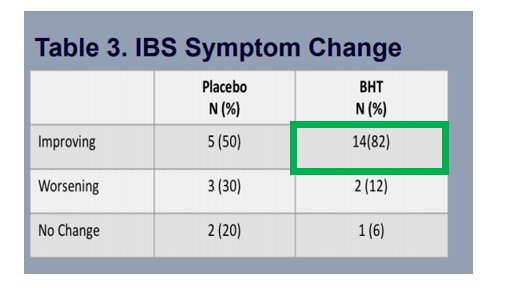
MCY-M11 gets me extremely excited as the very first data which is being obtained is showing that with a single low dose it stops cancer progression for up to 6 months with most side effects being Mild (Grade 1/2)
This disease control is shown in virtually every patient

MCY-M11 gets me extremely excited as the very first data which is being obtained is showing that with a single low dose it stops cancer progression for up to 6 months with most side effects being Mild (Grade 1/2)
This disease control is shown in virtually every patient


#MXCT 🧬
MCY-M11 is only the first generation (Least complicated) CAR T therapy MaxCyte subsidiary CARMA have produced
Many more optimisations/additions will likely now take place over the following years, similar to the evolution of Fate Therapeutics pipeline
MCY-M11 is only the first generation (Least complicated) CAR T therapy MaxCyte subsidiary CARMA have produced
Many more optimisations/additions will likely now take place over the following years, similar to the evolution of Fate Therapeutics pipeline

#MXCT 🧬
As these therapies are approved for medical use how will all these doses of each therapy be made?
By buying each and every machine/cassette from MaxCyte🔥
Not only will MaxCyte achieve revenues via this avenue they will also receive
-Milestones
-Royalties
💰💵💰💵

As these therapies are approved for medical use how will all these doses of each therapy be made?
By buying each and every machine/cassette from MaxCyte🔥
Not only will MaxCyte achieve revenues via this avenue they will also receive
-Milestones
-Royalties
💰💵💰💵


#MXCT 🧬
This is a platform technology that is growing “EXTRAORDINARILY” year on year
The adoption and sought after demand is likely to continue to increase YoY
Future revenue potential is mind blowing 🤯
This is a platform technology that is growing “EXTRAORDINARILY” year on year
The adoption and sought after demand is likely to continue to increase YoY
Future revenue potential is mind blowing 🤯

#MXCT 🧬
Another look at the partners which MaxCyte work with
$EDIT $5bn
$CRSP $12bn
KITE- Acquired by $GILD For $12bn
$SGMO $2.5bn
$ALLO $4bn
Another look at the partners which MaxCyte work with
$EDIT $5bn
$CRSP $12bn
KITE- Acquired by $GILD For $12bn
$SGMO $2.5bn
$ALLO $4bn

#MXCT 🧬
🔥BOLD words from the CEO of CRISPR Therapeutics $CRSP 🔥
Securing access to the
💥Leading “Cell and Gene Therapy” delivery platform 💥
🔥BOLD words from the CEO of CRISPR Therapeutics $CRSP 🔥
Securing access to the
💥Leading “Cell and Gene Therapy” delivery platform 💥

• • •
Missing some Tweet in this thread? You can try to
force a refresh