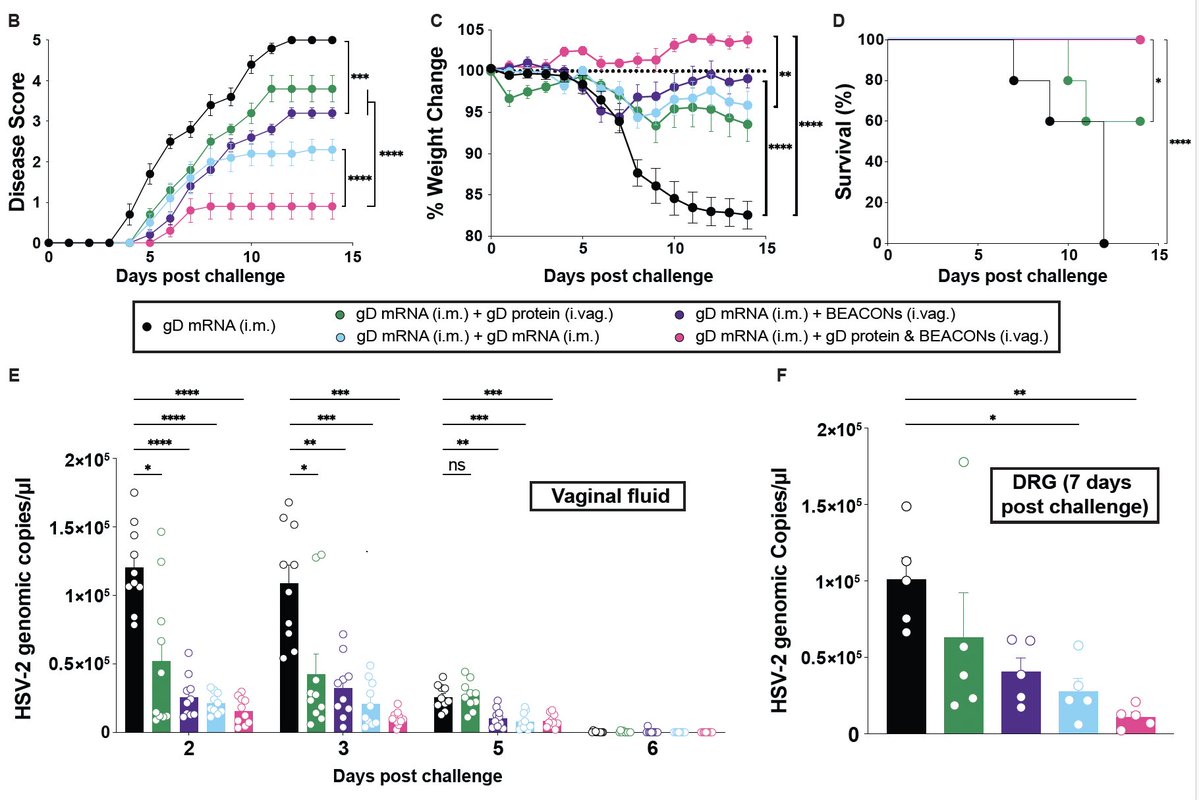
Are you confused about the role of type I interferons (IFN) in #SARS_CoV_2 infection?
Here is my speculation on what makes IFN-I helpful vs. harmful in #COVID19 patients - it comes down to timing and dose. A short thread.
All figures made by @BioRender & @annsea_park (1/n)
Here is my speculation on what makes IFN-I helpful vs. harmful in #COVID19 patients - it comes down to timing and dose. A short thread.
All figures made by @BioRender & @annsea_park (1/n)
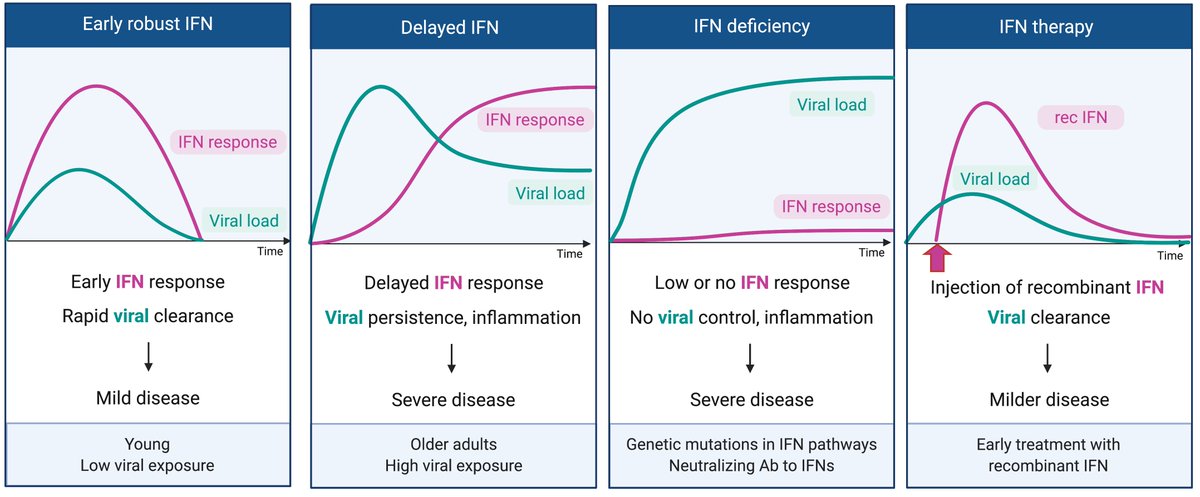
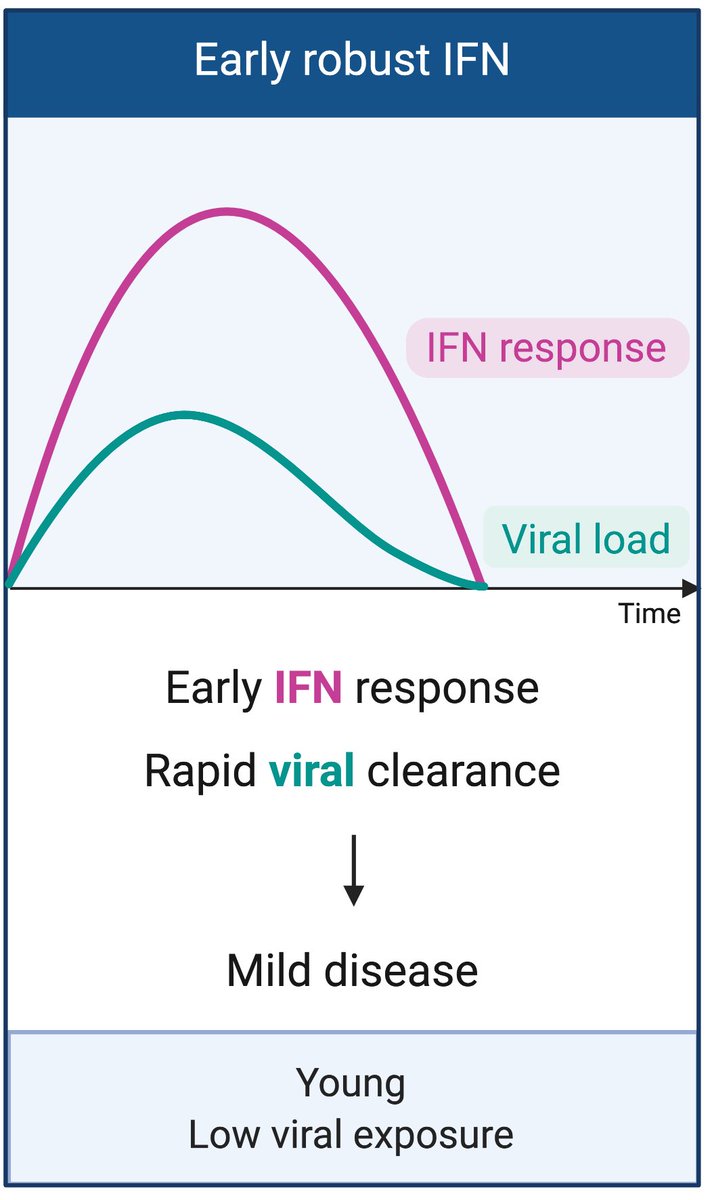
In the best case scenario, a rapid and robust induction of IFN-I should result in viral control and mild disease. This may happen in young people, or with low viral exposure settings. For a discussion we wrote, please read this.
(2/n)
cell.com/cell-host-micr…
(2/n)
cell.com/cell-host-micr…
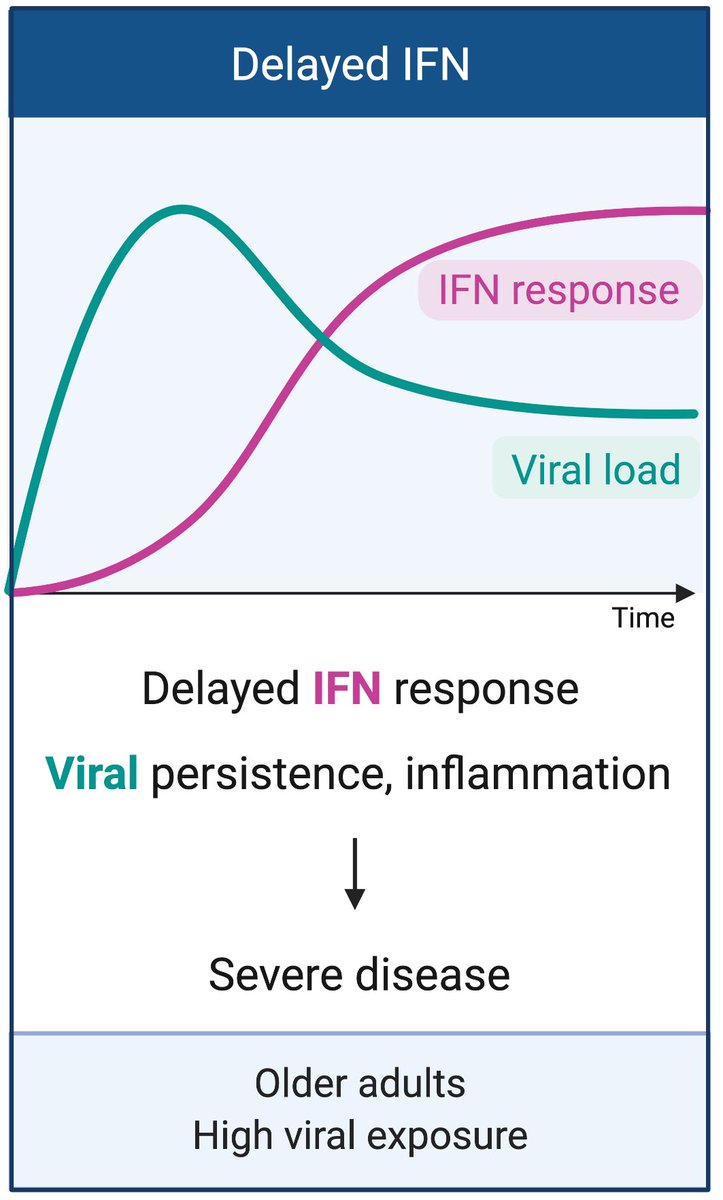
In older adults or after high dose viral exposure, impaired IFN response early during infection results in enhanced viral replication, and prolonged levels of IFN-I and IFN-III responses that could result in pathological consequences and severe disease. (3/n) 
Some papers supporting this scenario can be found here. (4/n)
immunology.sciencemag.org/content/5/49/e…
sciencedirect.com/science/articl…
rupress.org/jem/article/21…
nature.com/articles/s4158…
sciencedirect.com/science/articl…
science.sciencemag.org/content/369/65…
science.sciencemag.org/content/369/65…
stke.sciencemag.org/content/10/509…
immunology.sciencemag.org/content/5/49/e…
sciencedirect.com/science/articl…
rupress.org/jem/article/21…
nature.com/articles/s4158…
sciencedirect.com/science/articl…
science.sciencemag.org/content/369/65…
science.sciencemag.org/content/369/65…
stke.sciencemag.org/content/10/509…
Not all studies show this pattern. Here is a study that showed lower IFN-I levels in more severe COVID patients. It would be interesting to understand this difference. (5/n)
science.sciencemag.org/content/369/65…
science.sciencemag.org/content/369/65…
In the setting of host genetic mutations in viral sensors, signaling molecules, adaptors, transcription factors; or in older people with neutralizing antibodies to IFN-I, little to no IFN-I is available. Uncontrolled virus replication can lead to very severe COVID-19. (6/n) 
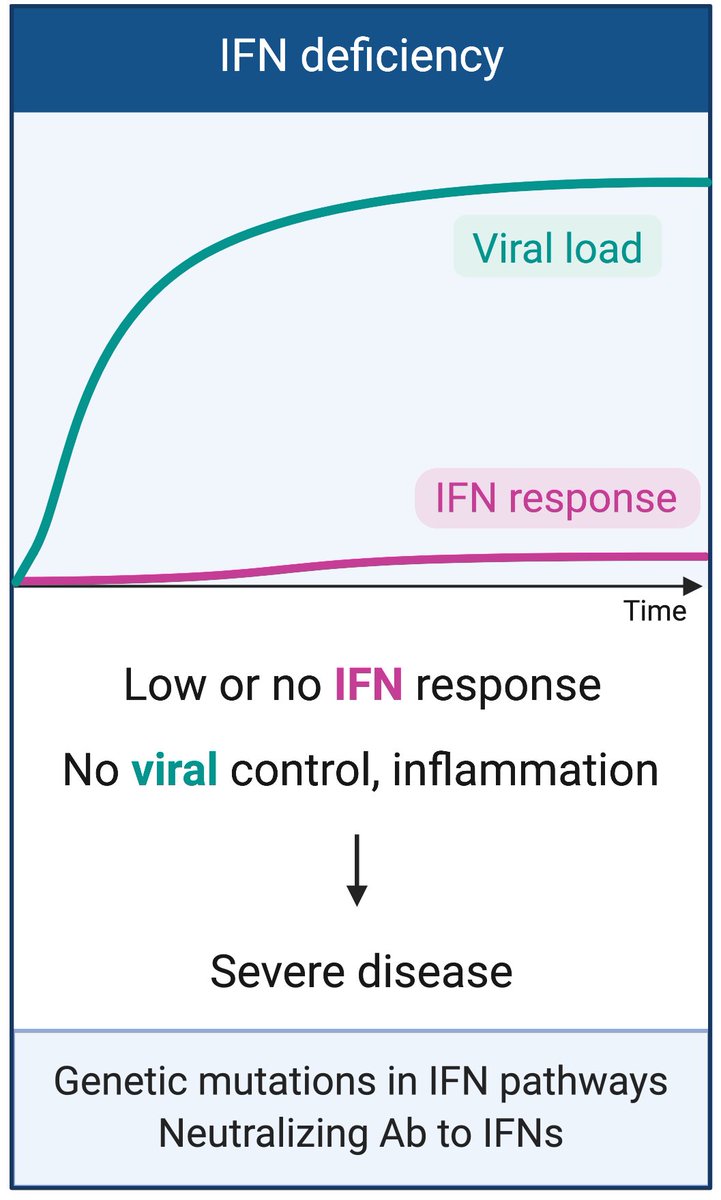
Papers supporting this scenario can be found here. (7/n)
science.sciencemag.org/content/early/…
science.sciencemag.org/content/early/…
jamanetwork.com/journals/jama/…
science.sciencemag.org/content/early/…
science.sciencemag.org/content/early/…
jamanetwork.com/journals/jama/…
Finally, recombinant IFN therapy, especially given early during infection, can shut down viral replication and promote recovery. Further, prophylactic treatment with rIFN might be useful in high risk groups. (8/n) 
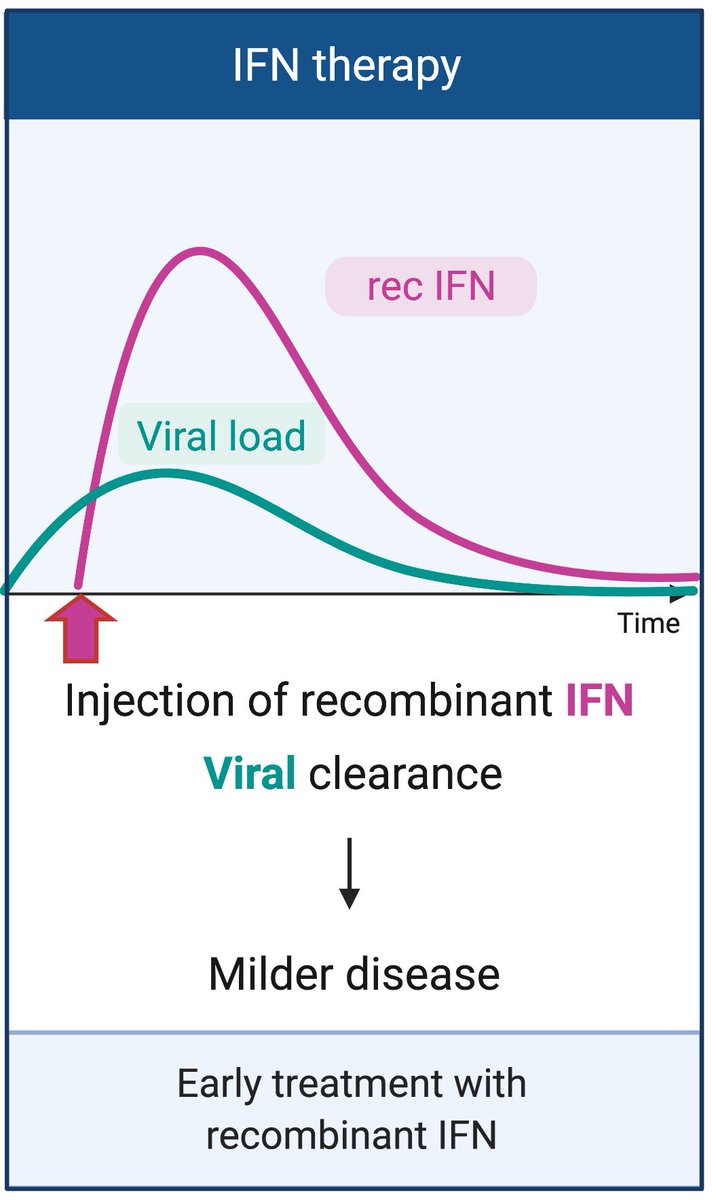
A number of clinical trials are under way to test whether IFN therapy can help #COVID-19 patients recover quickly from infection and disease. Thank you for reading! (End)
clinicaltrials.gov/ct2/results?co…
sciencemag.org/news/2020/07/c…
covid19treatmentguidelines.nih.gov/immune-based-t…
clinicaltrials.gov/ct2/results?co…
sciencemag.org/news/2020/07/c…
covid19treatmentguidelines.nih.gov/immune-based-t…
• • •
Missing some Tweet in this thread? You can try to
force a refresh