Next up, the “What’s Hot in Antifungals?” session by the ever-insightful @DavidAndes, @IDPharmacist, and Dr. Sharon Chen. There’s a lot of development in the antifungal sphere so strap in! @MSG_ERC @GermHunterMD @FungalDoc @GRThompson @IDSA @SIDPharm 1/
So what are the current gaps in available antifungals? Notable gaps include parenteral only, PK distribution to sanctuary sites/urine, toxicity, DDIs, and MDR pathogens (I’m looking at you, the fungus formerly known as S. prolificans). 2/
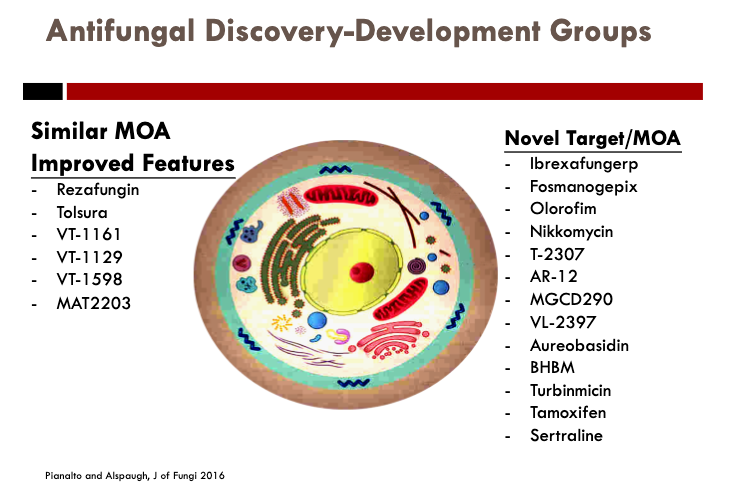
The novel antifungal pipeline in all of its glory, broken down into similar MOA with improvement in features vs. novel targets/mechanisms.
Some great reviews of newer agents for interested parties:
doi.org/10.1093/ofid/o…
doi.org/10.3390/jof601…
doi.org/10.3390/jof204…
3/
Some great reviews of newer agents for interested parties:
doi.org/10.1093/ofid/o…
doi.org/10.3390/jof601…
doi.org/10.3390/jof204…
3/
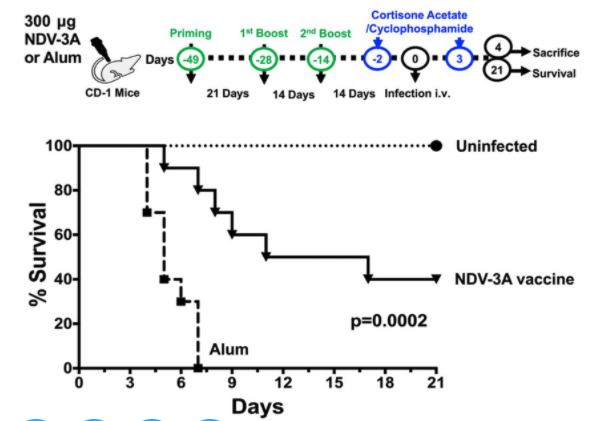
First, rezafungin (CD-101) (doi.org/10.1093/cid/ci…); modified anidulafungin improved stability in vivo, prolonged half-life (>100hrs); found NI to caspo in STRIVE candidemia/IC in response at d14, 30d mort, and safety; Phase 3s - ReSTORE IC/candidemia, ReSPECT IFI/PJP ppx. 4/ 







Ibrexafungerp (SCY-078) novel glucan synthase inhibitor (EC target, distinct site) self-promo (pubmed.ncbi.nlm.nih.gov/31342066/) IV/PO, QD dosing; Activity in common FKS mut Candida (including C. auris – pic2); Ongoing trials for VVC, combo IPA, refr IFI. Promise in VVC/IC/resistance. 5/ 







Otesseconazole (VT-1161), tetrazole (4Ns) improved target-spec; Broad, long T1/2, likely rVVC niche (basically fidaxomicin for VVC), but also onycho/tinea; Sibling agents VT-1598 <3 (Coccy, Asper, Zygo) + VT-1129 (Crypto) 6/ 



SUBA-itra (Tolsura), novel formulation/improved PK (pH-sensitive microparticle, 81% Cmin ≥ 1mcg/mL) (doi.org/10.1128/AAC.00…); P2 study endemic mycoses @MSG_ERC 7/ 

ORAL AMPHOTERICIN B (all caps=excitement ≠ typo) MAT-2203 encochleated form, release at site of infxn through macrophage engulfment (pic 2; 10.3390/jof6020066); Phase 1 safe, ongoing trials for mucocut cand., CrypMen 8/ 



Fomanogepix (APX-001), GPI inhibitor, broad-spectrum (including difficult hyalohypomycetes - 10.1128/AAC.01735-19; Zygos); Candida gap = C.krusei; IV/PO, CNS/eye penetration! Numerous trials, notably expanded access for fusariosis; P2 Candidemia w/o safety issues 9/ 





Olorofim (F901318) DHODH inhibitor (pyrimidine biosynth; hi spec. fung DHODH); IV/PO; noteworthy activity L. prolificans activity, +/- Fusarium, no Zygo/Crypto/Candida; Ongoing P2b MDR molds/difficult IFIs n=26 so far. 10/ 

Early clinical/pre-clinical novel MOA – novel modes of action/mechanisms 11/
Thank you @davidrandes for a magnificent summary of what's to come, making my way through @IDPharmacist's section and will post next! @IDSAInfo #IDWeek2020 #Antifungal #MedicalMycology

Thank you @davidrandes for a magnificent summary of what's to come, making my way through @IDPharmacist's section and will post next! @IDSAInfo #IDWeek2020 #Antifungal #MedicalMycology


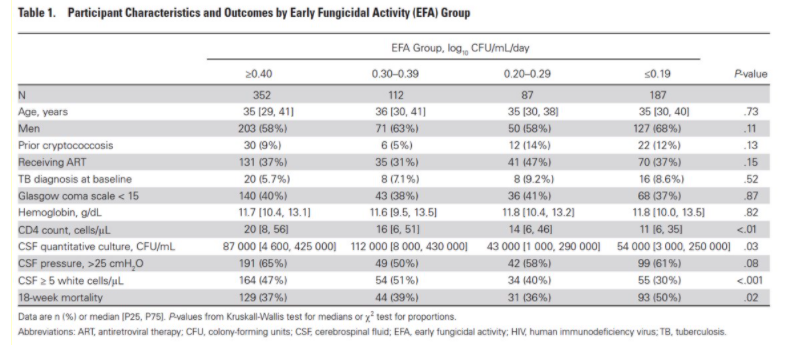
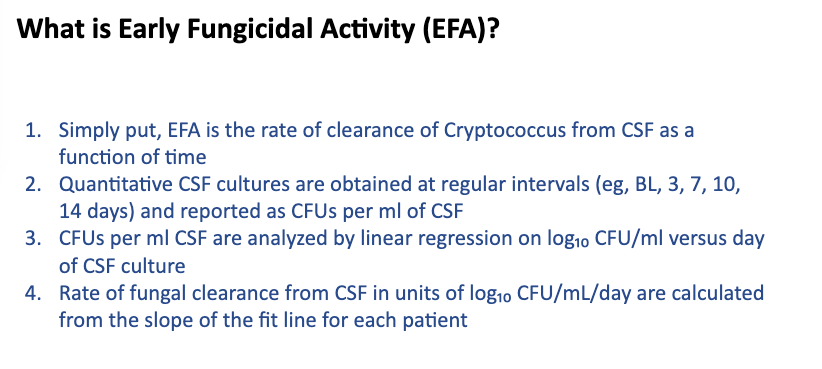
Next up, @IDPharmacist’s section on TDM (one of my favorite topics, right @erinmccreary?); Dr. J begins with a case (pic), IPA in BOLT, pre-exposed to azoles, post-txp IV posa 300mg, 1st level = 0.4!! (this is why you need to check lvls), inc dose 200mg Q12h IV rpt lvl 1.2. 12/ 

Challenges in PKPD are multi-factorial (host, PK inter/intra-variability, micro); Criteria for TDM 1) variability 2) defined exposure range 3) Narrow tx ind 4) defined sampling t (i.e. Cmin) 5) Assay time/accuracy. Guess how many boxes the azoles check off? (well most of ‘em) 13/ 

Hit ‘em with the comprehensive table! Take a second and look at this slide, notice how many gaps there are, we need real more data here. Also h/t @Russlewis_BO for this bad boy (ecil-leukaemia.com/telechargement…); Most PD targets for candidiasis 14/ 

Vori (the red-headed step-child of azoles), high variability (2C19 polymorph), narrow index, tox w/ Cmin ≥ 4.5-5, efficacy ≥ 1-1.5. I have this figure above my desk and reference frequently (Pic 1; from doi.org/10.1093/cid/ci…) 15/ 

Posa – Pseudohyperaldosteronism 2/2 inhibition of 11BHSD2/11B-OH->inc 11-deoxycortisol/corticosterone; read in our pubs here (Levels associated - doi.org/10.1093/cid/ci…; Management of PIPH doi.org/10.1093/jac/dk…; Poster 752!!!) @nguyenmvu_md @Grthompson @tjgintjee @Jobadd 16/ 

PKPD in special pops requires individualized approach, helpful table in pic; need more ECMO data; DDIs table with degree of CYP450/P-gp inhibition, save this one for later (actually, save this whole presentation for later) 17/ 



Is TDM performed needed? Yes, for most. We need more clinical/PKPD data to flesh out targets/toxicodynamic thresholds tho
Helpful resources:
doi.org/10.1007/s00134… (h/t @SIDPharm breakpoints @Ryankshields @ErinMcreary)
doi.org/10.1093/jac/dk… (TDM BSMM guidelines, 2013) 18/
Helpful resources:
doi.org/10.1007/s00134… (h/t @SIDPharm breakpoints @Ryankshields @ErinMcreary)
doi.org/10.1093/jac/dk… (TDM BSMM guidelines, 2013) 18/

What factors need to consider? 1) turn-around time 2) DOT 3) indication 4) likelihood of resistance
5) Host factors, serverity/degree of IS, organ fxn; Last the $1M question, “will it change management?” 19/
5) Host factors, serverity/degree of IS, organ fxn; Last the $1M question, “will it change management?” 19/
Pic says it all – 30% of levels run at UTHSC subtx (fun fact: lab director Nathan Widerhold was my prof); Optimizing subtx level tips vori – add omep, inc freq > dose; posa – DR tabs slight boost from food (less than susp), NEVER use susp (my own take), consider IV, dosing 20/ 

High levels – drop dose, hold if super-high, restart lower + TDM; Back to case (pic 1) posa IV 200 q8 lvl 3.1-> 300 PO BID = 1.7->2.3 later, improved/discharged
Future steps (pic 2) need to define targets/improve assay availability/dosing, novel agents might lessen TDM need 21/
Future steps (pic 2) need to define targets/improve assay availability/dosing, novel agents might lessen TDM need 21/

#IDWeek2020 Part 3 – Emerging Antifungal Resistance (AFR) by Dr. Sharon Chen; Starting with defining resistance itself (inherent v acquired, predictable v not); 3 pillars 1) host 2) bug 3) drug (“Doug, bug, and drug” as I say) 22/ 

The AFR country club – C. glab (now “Nakaseomyces glabrata” DOI: 10.1128/JCM.01811-20), C. auris, A. fumigatus sensu stricto, Lometospora, Fusarium, uncommon yeasts; Honorable mention, Zygos. 23/ 

Inherited/acquired resistance yeasts – C. glab/C. krus (Now P. kudriavzevii) w/ inh. resistance to azoles, C. auris w/ azole/AmB, all 3 can acquire EC resistance via FKS mutations; non-Candidas inherent EC resistance can be present; More (DOI: 10.4274/tjh.2018.0007, pic 2) 24/ 



Mo(u)lds next – Asp genus inherent fluc-R, some spp. AmB-R as well, see pic 2 above for details (hint: Lomentospora is a jerk); L. prolificans stands alone from other Scedo spp., problematic in HONC pts/SOT, don’t forget S. apio though! (can affect any body site – pic 2) 25/ 



How to treat? In AUS vori=DOC, low GM MICs v. POS/AmB; Potential options? Enter miltefosine (cases of success as salvage for L. prol, in vitro synergy w/ VOR/POS) uncertain in vivo impact (typical)
BONUS: impavido.com/contact if need miltef for VL or PAE @infectiousRamee 26/
BONUS: impavido.com/contact if need miltef for VL or PAE @infectiousRamee 26/

Combo tx? Vor/terb in vitro synergy (85% of L. prol strains, 16-fold lower vori MIC); Other AmB or vor + EC, data scarce; Case series n=41 L.prol (66% hem, 61% disseminated, att rmort 51% ☹), combo tx a/w survival, terbinafine a/w higher success (doi.org/10.1111/myc.13…) 27/ 

OLOROFIM (my favorite) – favorable in vitro (pic1), await P2b data; Azole res. in Aspergillus can be acquired via environ vs. prev exposure to drugs; Mutations in CPA (azole-exposed) = G54/M220/G138 common sites -> chngs in access channels= x-resistance in class; 28/ 

Environmental = cyp51A (target for azoles) overexpression ± TR duplication in promoter region; common mutants listed, note 10% no mutation in cyp51A target site, likely efflux; nice review (doi: 10.1101/cshperspect.a019752) if you’re interested. 29/ 

Tips!! 7% incidence of azole resist in Cx (+) pts globally (local variance tho); 64-71% of azole resist. IA HAVE NO PREVIOUS EXPOSURE, i.e. Environ; Clinical decision making tips for IA tx (pic1)
BONUS: Personal favorite – bIFI mgmt. in HONC (pic2; DOI: 10.1093/cid/ciy473) 30/

BONUS: Personal favorite – bIFI mgmt. in HONC (pic2; DOI: 10.1093/cid/ciy473) 30/


If azole resistance > 10%, vori mono ≠ 1st line, combo w/ EC (per Marr et al classic: doi.org/10.7326/M13-25…) or VOR/AmB (less enthused here); azole resist 5-10% What to do?, it depends on situation! 31/
Final thoughts: C. auris MDR/XDR properties, IP difficulties, resistance is clone/clade-specific (pic1); CDC proposed bkpts/EUCAST ECVs in table (pic2)
BONUS: CMR DOI: 10.1128/CMR.00029-17) 32/

BONUS: CMR DOI: 10.1128/CMR.00029-17) 32/


≥1 class resist ~75%, ≥2 classes ~20%, pan-resistance <5% %, n=848 CDC surveillance; Must perform susceptibility, variable resistance; Biofilms drive resistance, ibrexafungerp might be useful (see again pubmed.ncbi.nlm.nih.gov/31342066/ biofilm section); 33/
How to treat C. auris? Need to know local epi/clades, resistance; ECs remain DOC, posa/Isa low MICs as well, Ibrexafungerp very promising in vitro + biofilm activity even in EC-R strains (poster again). 34/ 

Summary – AFR occurs, may not be predictable (know ur local epi), test isolates if R is hi! New tx becoming available (see above); Some rapid dx tests available, barrier need to have defined targets, could miss.
What a great talk, go listen! 35/35 @IDSAInfo @SIDPharm @MSG_ERC
What a great talk, go listen! 35/35 @IDSAInfo @SIDPharm @MSG_ERC
• • •
Missing some Tweet in this thread? You can try to
force a refresh