1/14
Why is secondary dengue infection more likely to cause hemorrhagic fever than primary infection?
Not all infections confer immunity, but why would prior exposure lead to WORSE outcomes?
To answer these questions, we'll need to discuss "Original Antigenic Sin".
Let's go!
Why is secondary dengue infection more likely to cause hemorrhagic fever than primary infection?
Not all infections confer immunity, but why would prior exposure lead to WORSE outcomes?
To answer these questions, we'll need to discuss "Original Antigenic Sin".
Let's go!
2/
Dengue is caused by any of the four dengue virus serotypes (DENV 1-4).
Dengue hemorrhagic fever (DHF) is a severe form of dengue characterized by vascular leakage, hemorrhage, and thrombocytopenia.
This can lead to organ failure and death.
apps.who.int/iris/bitstream…
Dengue is caused by any of the four dengue virus serotypes (DENV 1-4).
Dengue hemorrhagic fever (DHF) is a severe form of dengue characterized by vascular leakage, hemorrhage, and thrombocytopenia.
This can lead to organ failure and death.
apps.who.int/iris/bitstream…
3/
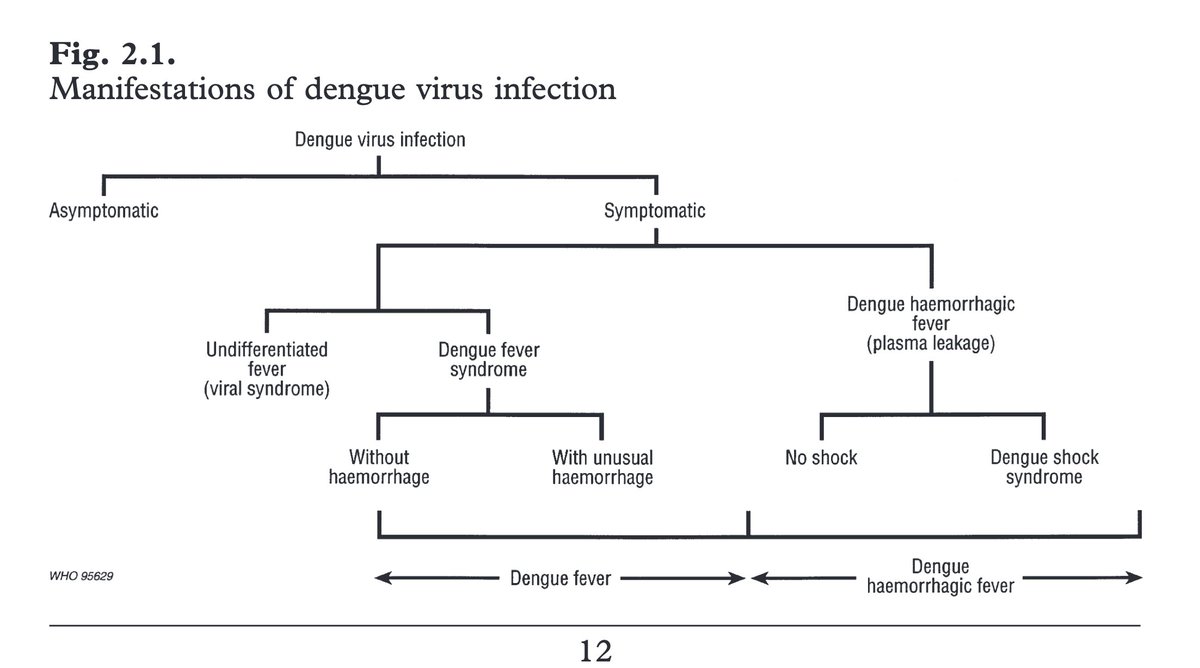
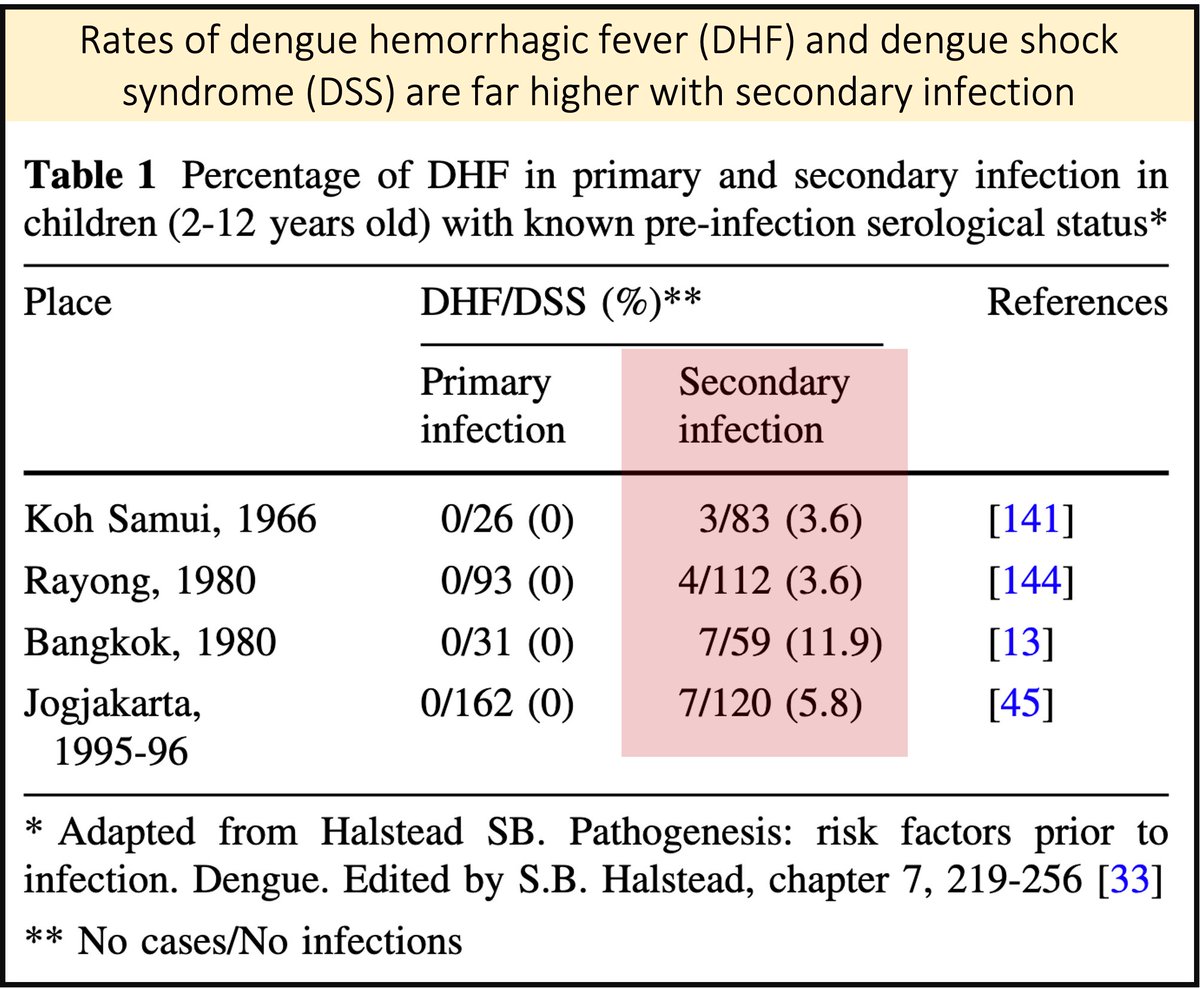
The biggest risk factor for DHF is secondary infection (i.e. patients with DHF have been infected with dengue once before).
Multiple cohorts have shown that DHF is rare the first time someone is infected.
pubmed.ncbi.nlm.nih.gov/23471635/
The biggest risk factor for DHF is secondary infection (i.e. patients with DHF have been infected with dengue once before).
Multiple cohorts have shown that DHF is rare the first time someone is infected.
pubmed.ncbi.nlm.nih.gov/23471635/
4/
Importantly, the second infection must be from a different serotype than the one that caused the primary infection (aka "heterotypic infection").
The order of infection most likely to cause DHS is:
◾️Primary DENV-1, followed by
◾️Secondary DENV-2
pubmed.ncbi.nlm.nih.gov/6496446/
Importantly, the second infection must be from a different serotype than the one that caused the primary infection (aka "heterotypic infection").
The order of infection most likely to cause DHS is:
◾️Primary DENV-1, followed by
◾️Secondary DENV-2
pubmed.ncbi.nlm.nih.gov/6496446/

5/
To understand why heterotypic secondary infection is a risk factor for DHS we must discuss "Original Antigenic Sin"(OAS).
OAS was proposed in 1953 by Thomas Francis as a way to explain unexpected patterns of antibody response to influenza.
pubmed.ncbi.nlm.nih.gov/13109114/
To understand why heterotypic secondary infection is a risk factor for DHS we must discuss "Original Antigenic Sin"(OAS).
OAS was proposed in 1953 by Thomas Francis as a way to explain unexpected patterns of antibody response to influenza.
pubmed.ncbi.nlm.nih.gov/13109114/

6/
Francis and others observed that sequential exposure to new viral variants induces preferential antibody response to the FIRST virus strain encountered.
As some have put it, someone's "First Flu is Forever".
jstor.org/stable/985534?…
pubmed.ncbi.nlm.nih.gov/27846592/
Francis and others observed that sequential exposure to new viral variants induces preferential antibody response to the FIRST virus strain encountered.
As some have put it, someone's "First Flu is Forever".
jstor.org/stable/985534?…
pubmed.ncbi.nlm.nih.gov/27846592/
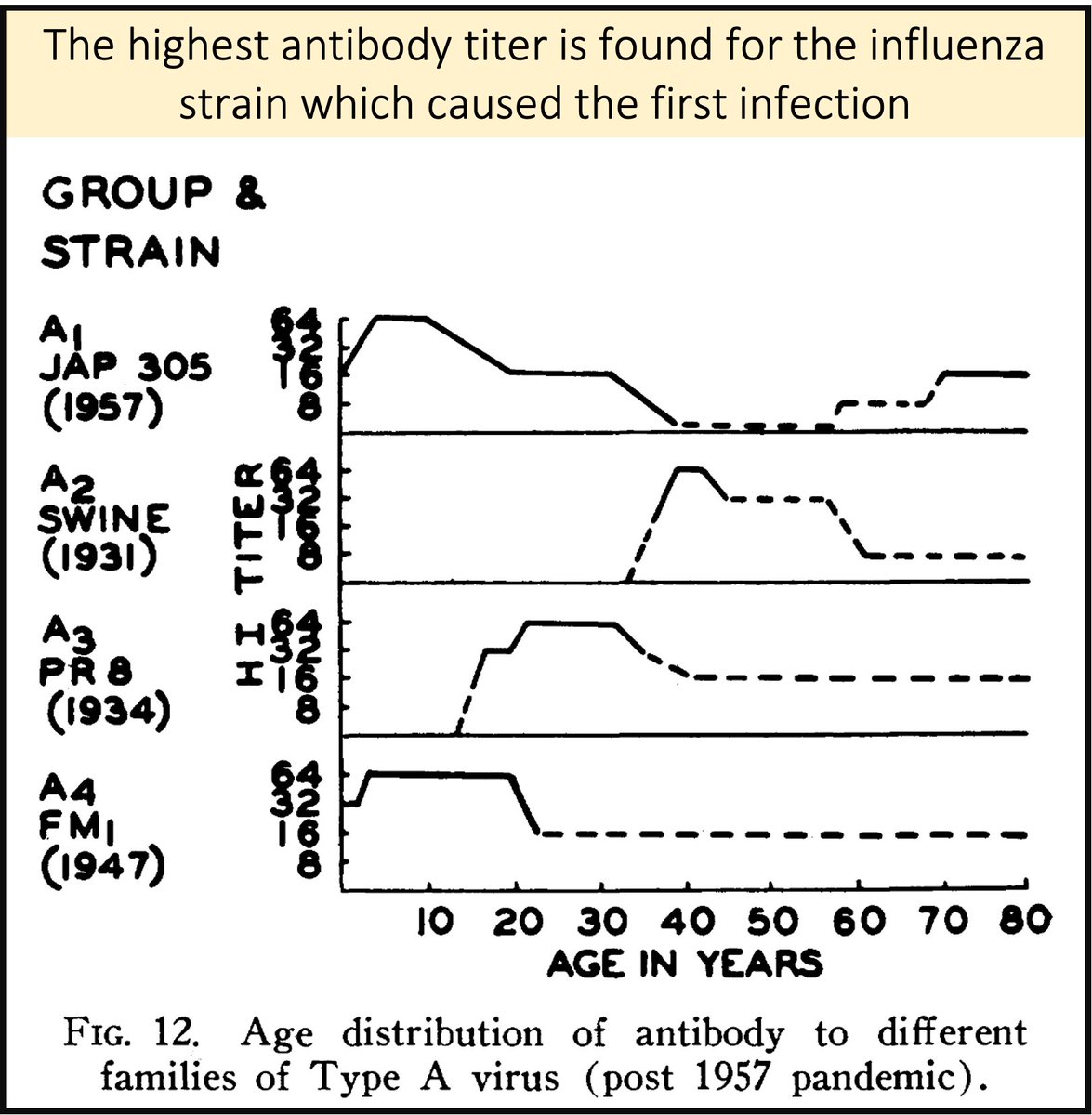
7/
Our immune system will respond to subsequent - heterotypic - infections by creating the best response to someone's "original" serotype (even though this isn't the current one).
A lesser response is seen against the newer variant.
pubmed.ncbi.nlm.nih.gov/13345965/
Our immune system will respond to subsequent - heterotypic - infections by creating the best response to someone's "original" serotype (even though this isn't the current one).
A lesser response is seen against the newer variant.
pubmed.ncbi.nlm.nih.gov/13345965/
8/
In influenza, repeated heterotypic infection leads to a POOR response to new strains. In dengue, heterotypic infection leads to a WORSE response (i.e. DHS).
These different responses can be explained by something called antibody-dependent enhancement.
pubmed.ncbi.nlm.nih.gov/21760609/
In influenza, repeated heterotypic infection leads to a POOR response to new strains. In dengue, heterotypic infection leads to a WORSE response (i.e. DHS).
These different responses can be explained by something called antibody-dependent enhancement.
pubmed.ncbi.nlm.nih.gov/21760609/
9/
Antibody-dependent enhancement (ADE) proposes that at some concentrations, heterotypic antibodies bind but do not neutralize virions of the secondary infecting viral type.
ADE requires enough antibody to bind but not so much as to promote killing.
pubmed.ncbi.nlm.nih.gov/20153282/
Antibody-dependent enhancement (ADE) proposes that at some concentrations, heterotypic antibodies bind but do not neutralize virions of the secondary infecting viral type.
ADE requires enough antibody to bind but not so much as to promote killing.
pubmed.ncbi.nlm.nih.gov/20153282/

10/
That a narrow range of anti-dengue antibody titers is a risk factor for DHS was shown in a study of 6684 children with antibodies titers against Dengue.
<1:21 = mildly increased mortality
1:21-1:80 = HIGHEST mortality
>1:1280 = lowest mortality
pubmed.ncbi.nlm.nih.gov/29097492/
That a narrow range of anti-dengue antibody titers is a risk factor for DHS was shown in a study of 6684 children with antibodies titers against Dengue.
<1:21 = mildly increased mortality
1:21-1:80 = HIGHEST mortality
>1:1280 = lowest mortality
pubmed.ncbi.nlm.nih.gov/29097492/
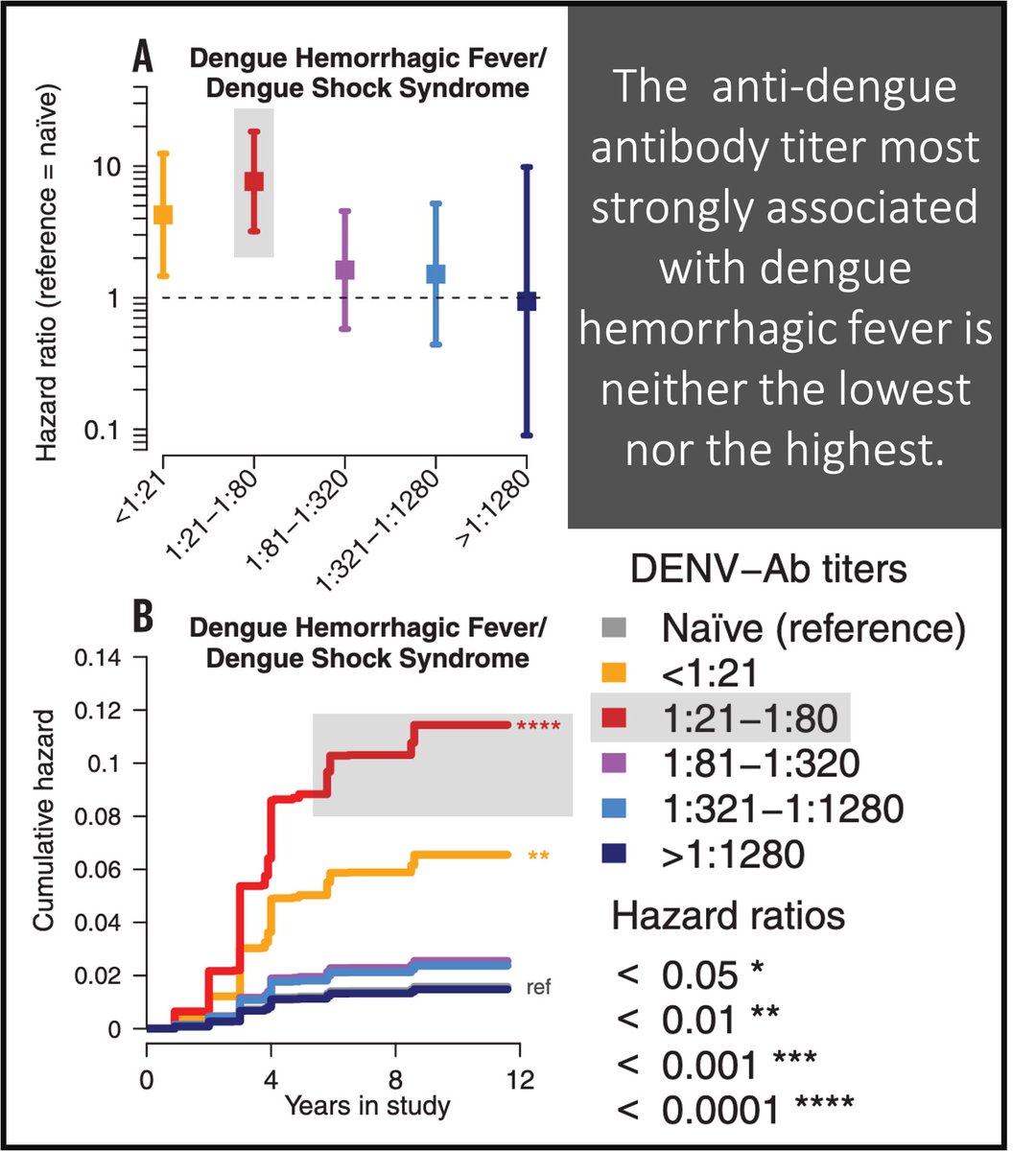
11/
How does ADE work in dengue?
Virion/antibody complexes are recognized by Fc-receptors that facilitate virus entry and replication in target cells.
Antibodies help the virus "sneak" into cells!
Result: high viral loads and increased risk of DHF.
pubmed.ncbi.nlm.nih.gov/21760609/
How does ADE work in dengue?
Virion/antibody complexes are recognized by Fc-receptors that facilitate virus entry and replication in target cells.
Antibodies help the virus "sneak" into cells!
Result: high viral loads and increased risk of DHF.
pubmed.ncbi.nlm.nih.gov/21760609/
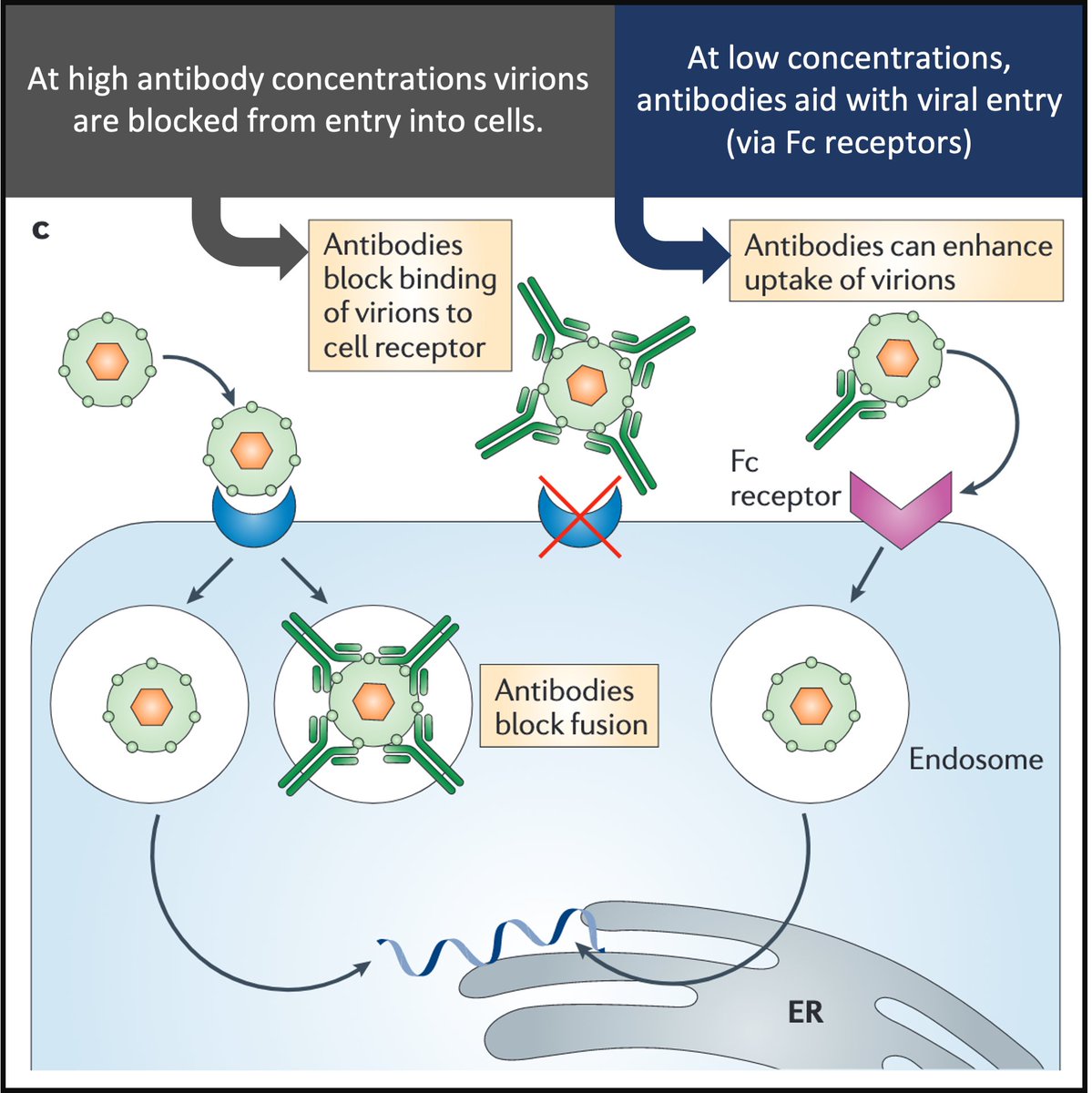
12/
OAS and ADE are seen in some (e.g. Zika), but not all (e.g. yellow fever) flaviviruses.
Why not yellow fever? It has just one serotype!
These phenomena have also been described in other viruses (e.g. HIV) and even bacteria (e.g. malaria).
pubmed.ncbi.nlm.nih.gov/28479213/
OAS and ADE are seen in some (e.g. Zika), but not all (e.g. yellow fever) flaviviruses.
Why not yellow fever? It has just one serotype!
These phenomena have also been described in other viruses (e.g. HIV) and even bacteria (e.g. malaria).
pubmed.ncbi.nlm.nih.gov/28479213/
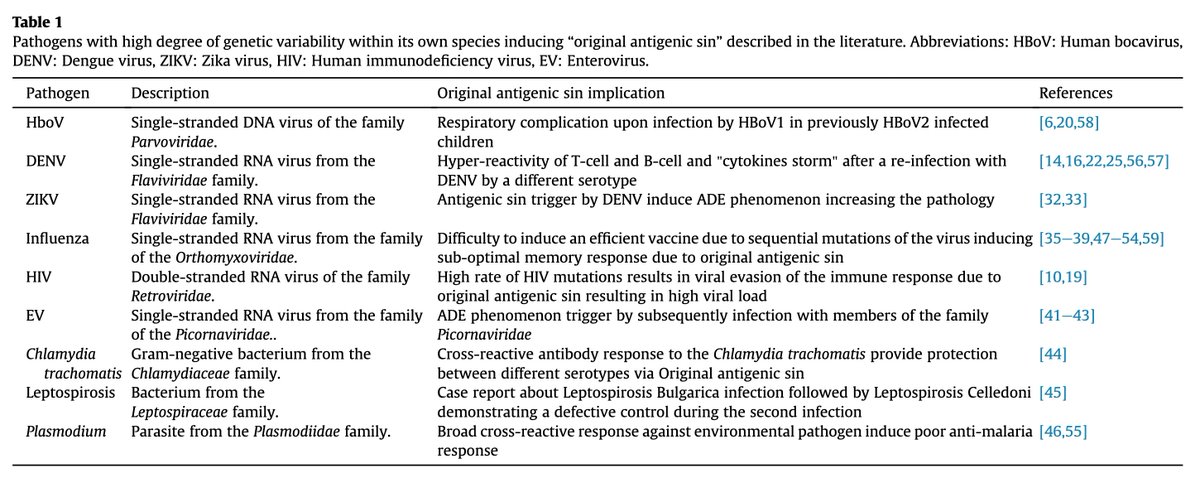
13/
Unsurprisingly, these mechanisms have also been offered as an explanatory model in COVID-19.
Given that multiple coronaviruses are circulating, could priming by one lead to ADE with SARS-CoV-2?
pubmed.ncbi.nlm.nih.gov/32582200/
pubmed.ncbi.nlm.nih.gov/32092539/
pubmed.ncbi.nlm.nih.gov/32908214/ (pic)
Unsurprisingly, these mechanisms have also been offered as an explanatory model in COVID-19.
Given that multiple coronaviruses are circulating, could priming by one lead to ADE with SARS-CoV-2?
pubmed.ncbi.nlm.nih.gov/32582200/
pubmed.ncbi.nlm.nih.gov/32092539/
pubmed.ncbi.nlm.nih.gov/32908214/ (pic)

14/14 SUMMARY
➢ The greatest risk factor for dengue hemorrhagic fever is secondary infection with a different serotype
➢ Original antigenic sin and antibody dependence enhancement may explain this finding
➢ Both phenomena are seen in other pathogens with antigenic variability
➢ The greatest risk factor for dengue hemorrhagic fever is secondary infection with a different serotype
➢ Original antigenic sin and antibody dependence enhancement may explain this finding
➢ Both phenomena are seen in other pathogens with antigenic variability
• • •
Missing some Tweet in this thread? You can try to
force a refresh