One question I had with the Pfizer results - is it possible that all cases were in younger (risk-taking) adults? Unlikely, but I couldn't tell. So it's great to see a breakdown of those 95 cases across different subpopulations. Representation is important! 6/10 



Nice safety profile, and participants will continue to be followed. Remember - evaluation of vaccine safety never stops, even after vaccines are approved and deployed. We have an adverse event reporting system to continually monitor all vaccines. 7/10 

Another key point - the Moderna vaccine has been formulated to not require the same intensive cold chain as Pfizer's vaccine (mRNA is unstable!). This has the potential to greatly simplify the logistics of rolling out the vaccine. 8/10
https://twitter.com/bachyns/status/1328331196313587713?s=20
What I am still very interested in seeing as the trial continues:
- Data on infection as measured by seroconversion to a non-spike protein (this is a secondary endpoint in Moderna's trial)
- Data collected over time on the durability of the effect
9/10
- Data on infection as measured by seroconversion to a non-spike protein (this is a secondary endpoint in Moderna's trial)
- Data collected over time on the durability of the effect
9/10

I am so proud of the scientific community for their determination and innovation. Given our current national situation, I think we are all grateful for the good news!
What questions do you have about the results? (I'll check in throughout the day.) 10/END
What questions do you have about the results? (I'll check in throughout the day.) 10/END
For some reason Twitter didn’t include Tweet 5/10 from my Moderna vaccine thread today. Sharing again it hopes it can be seen!
https://twitter.com/nataliexdean/status/1328356813557944320
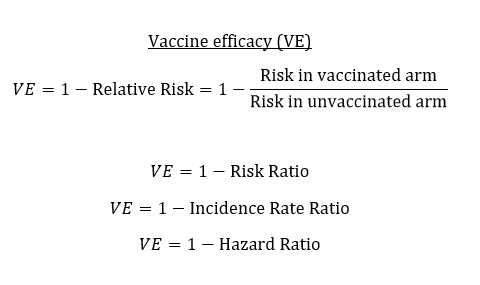
(Take two) For some reason, I can see my 5th tweet in this thread, but no one else can. (Is this Twitter jail?) Here is a screenshot! 

• • •
Missing some Tweet in this thread? You can try to
force a refresh