
THREAD:
Incredible new study results for rapid antigen test by @AbbottNews BinaxNOW
In KIDS and adults; with symptoms and fully asymptomatic.
Sensitivity:
100% at Ct <25
98.6% at Ct <30
Specificity 99.4% - 100%
1/
mass.gov/doc/binaxnow-a…
Incredible new study results for rapid antigen test by @AbbottNews BinaxNOW
In KIDS and adults; with symptoms and fully asymptomatic.
Sensitivity:
100% at Ct <25
98.6% at Ct <30
Specificity 99.4% - 100%
1/
mass.gov/doc/binaxnow-a…
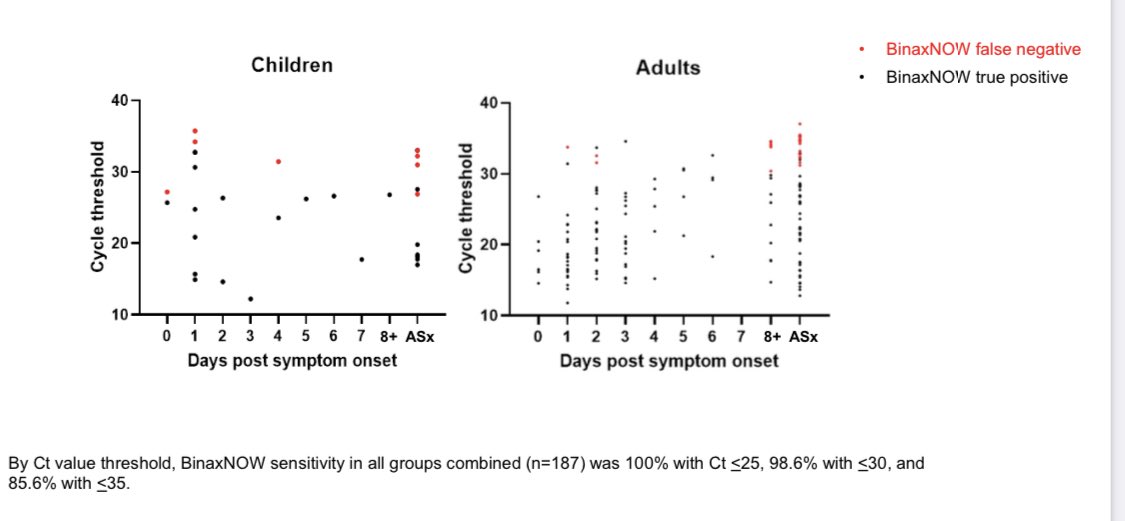
The study above, by @NiraPollock and others, is incredibly important because it proves that these tests do not care about your symptoms, they only care about the virus. And that they work in kids as well as adults.
The former issue is critically important...
2/
The former issue is critically important...
2/
Currently @US_FDA requires that for these tests to be used at home OTC, they need to demonstrate explicitly that they work in asymptomatic people.
But this is completely unnecessarily burdensome - plus finding asymptomatic people shedding virus is very difficult!
3/
But this is completely unnecessarily burdensome - plus finding asymptomatic people shedding virus is very difficult!
3/
The study shows the test works in symptomatic and asymptomatic people to catch the virus. This makes sense!
The FDA should immediately stop requiring rapid test companies to redemonstrate the physiology of #COVID19. We know the virus grows in asymptomatics.
4/
The FDA should immediately stop requiring rapid test companies to redemonstrate the physiology of #COVID19. We know the virus grows in asymptomatics.
4/
If FDA relaxed this requirement - trialing specifically in contagious asymptomatics (hard to find!) - rapid test cos could greatly accelerate their EUA applications.
This would increase home access to these and similar rapid antigen tests and help control this virus.
5/5
This would increase home access to these and similar rapid antigen tests and help control this virus.
5/5
Unfortunately this specific test is now going to be selling for $25 instead of $5. Which is truly unfortunate. But the study shows that rapid antigen paper strip tests can do exactly what we’ve been saying they can do... catch infectious people quickly and cheaply.
• • •
Missing some Tweet in this thread? You can try to
force a refresh




