
Let me just try to preempt all the BS (bad science) we will hear about hypothetical superiority of COVAXIN against variants vs Novavax. A thread 🧵 1/ 

Novavax is proven efficacious against OG COVID-19 and B.1.1.7 in a Phase 3 clinical trial in U.K. We should soon have the data in a preprint / peer reviewed publication. The confidence in the results will increase as more cases occur. 2/
Novavax has a Phase 2 trial in South Africa with an efficacy endpoint. That trial reported efficacy albeit reduced against B.1.351. The number of participants in the study was small, number of cases were low and therefore confidence in these results in lower than we need. 3/
However other vaccines have neutralization data from in vitro experiments in Petri dishes in labs. Novavax actually has clinical trial data. Here’s an image that stratifies the level of available evidence of various vaccines against SARS-CoV-2 variants from various vaccines 4/ 
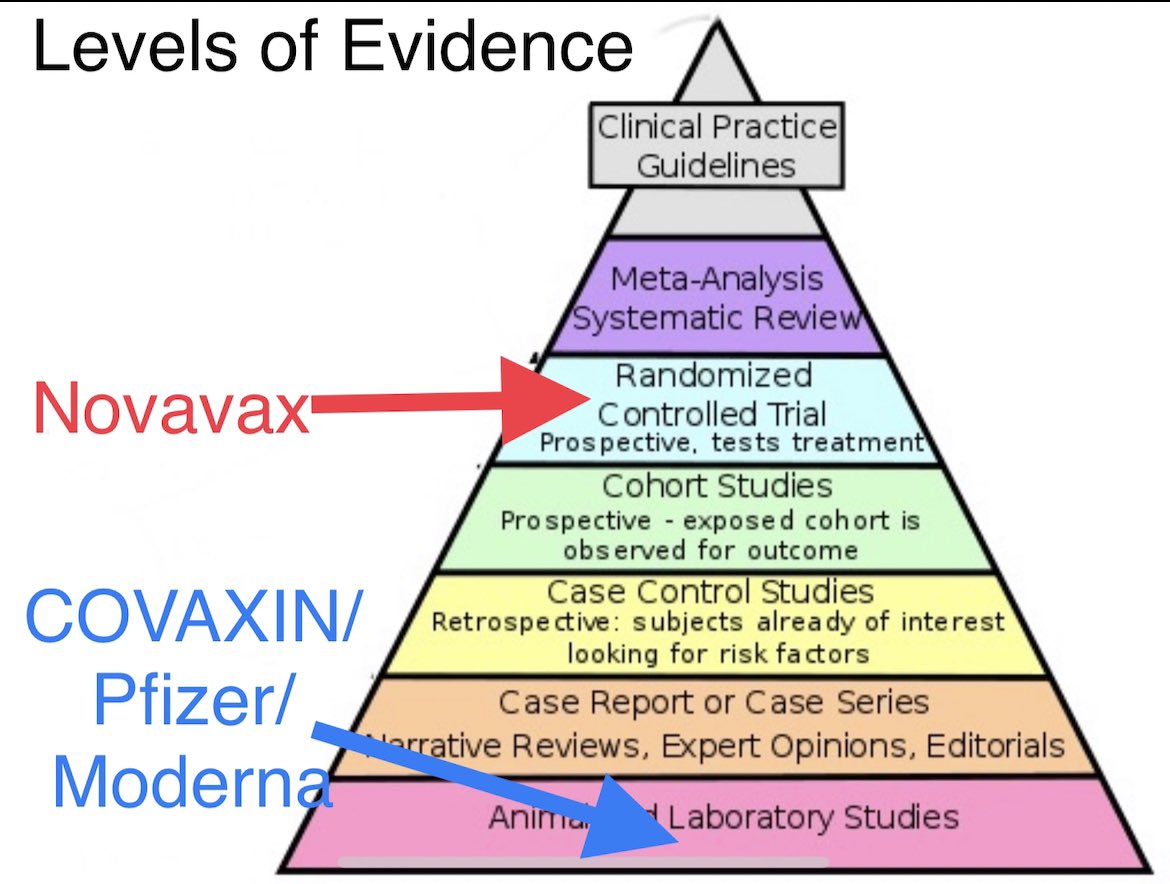
Novavax has Ph3 efficacy data from U.K. for B.1.1.7 & Ph2 efficacy data from SA for B.1.351. The number of participants & events in the Ph2 were small and the confidence intervals were wide. BUT clinical trials are at top of the evidence pyramid, lab studies are at bottom 5/ 
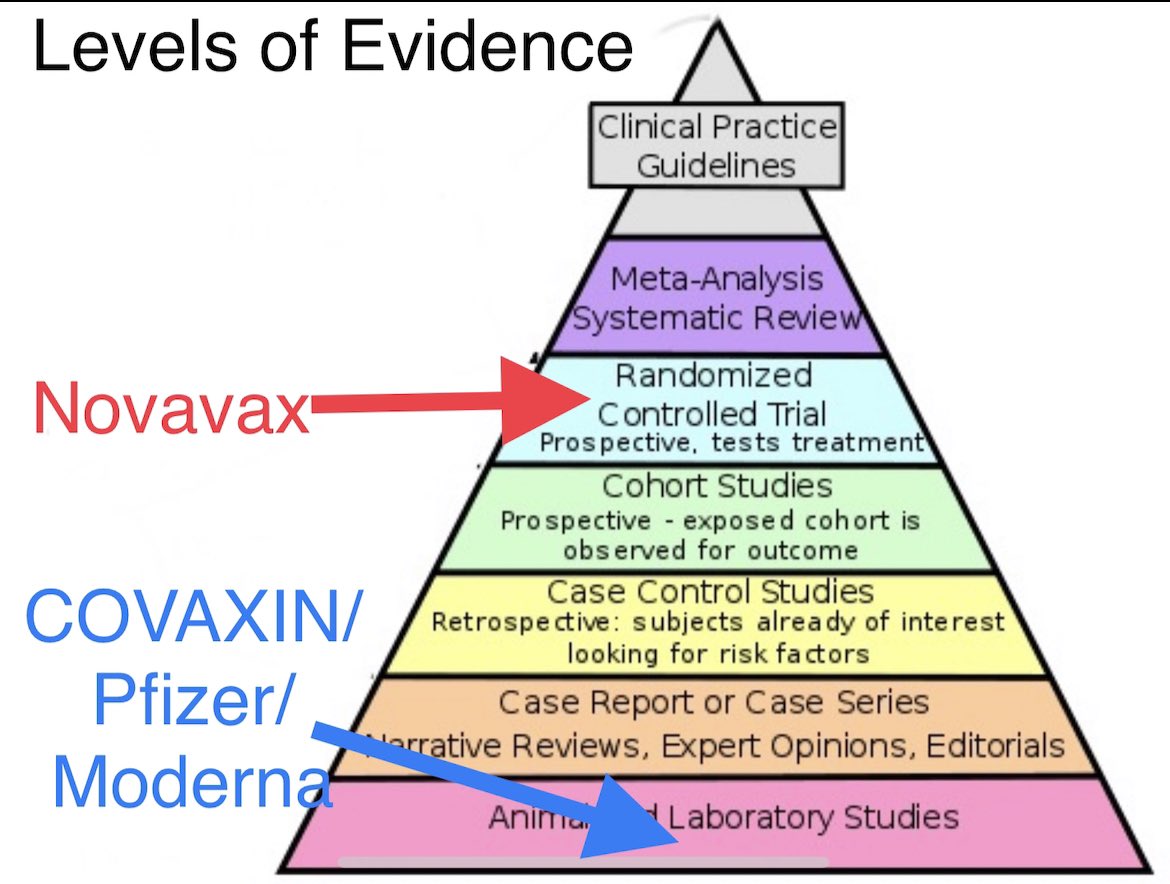
There’s valid concern that Novavax’s COVID-19 vaccine candidate doesn’t work well against B.1.351. While we don’t know the exact efficacy we know that there is some efficacy against symptomatic COVID-19 & hopefully more than that against severe COVID-19 caused by B.1.351 6/
There’s been this narrative that whole virus inactivated vaccines will provide better protection against variants. That’s categorically false. There’s a hypothesis that COVAXIN might provide better protection. NOT PROVEN. It may offer zero protection. We don’t KNOW. 7/
It is true that Novavax appears to offer less protection against B.1.351 variant first detected in SA. We’re not sure of the exact reduction in efficacy but this data is far superior to data for other vaccines. Can’t assume protection based on in vitro neutralization data. 8/
It’s very likely that other vaccines will have similar reductions in efficacy but we aren’t sure. This is a hypothesis that needs to be established in a clinical trial. Janssen has trials in US, SA, Brazil. Very interesting and important data coming very soon. 9/
We do know that Novavax vaccine produced incredibly high titers of neutralizing antibodies and there was still a decrease in efficacy against B.1.351 (Caveat neutralizing antibody tests are not standard across labs, this result could be due to low numbers, other reasons) 10/
It is unfortunate that some are putting Novavax down for lowered efficacy in a clinical trial while celebrating in vitro lab experiments for other vaccines. Very strange that COVAXIN (zero efficacy data) is said to be more effective against variants. No proof! 11/
It’s entirely possible that the facts and figures will change when we see more data, but right now Novavax looks like the best vaccine for India. The Ph3 for COVISHIELD was very messy, very few participants above 55 years, efficacy is unclear. COVAXIN has no Ph3 efficacy data 12/
Novavax will be manufactured in India as COVOVAX by Serum Institute. It is suitable for our cold chain logistics (2-8•C). It is very scalable (billions of doses). It has Ph3 efficacy and safety data. Can we expedite Novavax’s restricted EUA in clinical trial mode 😉 13/
Caveats
This is based on press release
Need to see preprint (incoming) & need peer review
Need safety data
Interim analysis has lower events and wider confidence intervals so let’s wait for more events
Efficacy against B.1.351 is from small Ph2
nAb assays aren’t standardized 14/
This is based on press release
Need to see preprint (incoming) & need peer review
Need safety data
Interim analysis has lower events and wider confidence intervals so let’s wait for more events
Efficacy against B.1.351 is from small Ph2
nAb assays aren’t standardized 14/

• • •
Missing some Tweet in this thread? You can try to
force a refresh




