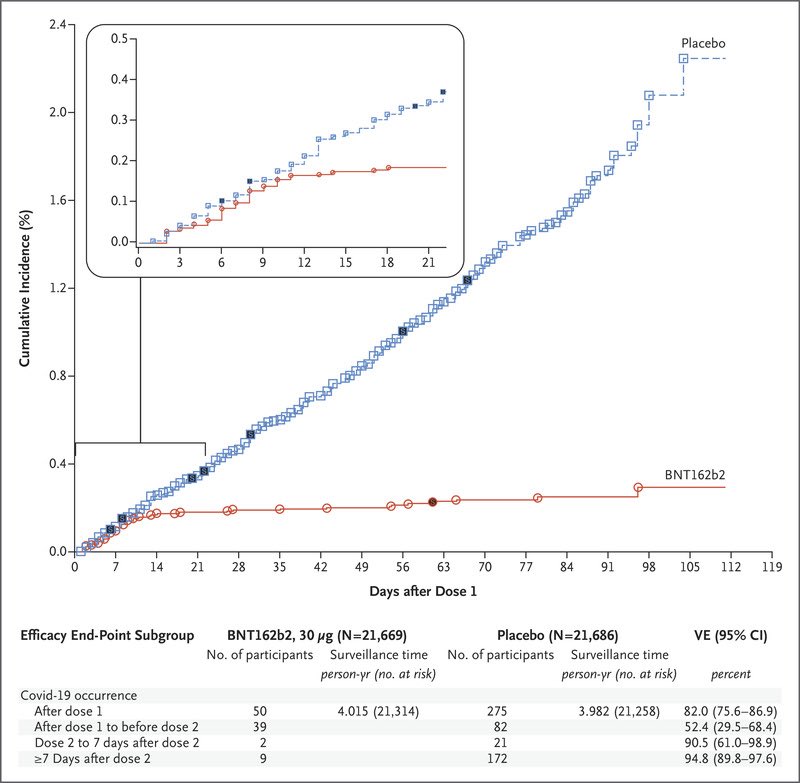
A thread on how the UK strategy relating to Pfizer vaccine rollout (i.e. 12 weeks between doses, rather than the 3 weeks followed in the Phase 3 clinical trial), places the most vulnerable in society, at PARTICULAR risk.
#FAO @JDBakewell
#FAO @JDBakewell
I was looking through the FDA website (as one does, for...um, FUN) for documents related to the Pfizer vaccine.
I found a briefing document, which has significant implications for the UK Pfizer strategy...and by "strategy", I mean, "reckless gamble". 🙄
fda.gov/media/144246/d…
I found a briefing document, which has significant implications for the UK Pfizer strategy...and by "strategy", I mean, "reckless gamble". 🙄
fda.gov/media/144246/d…
On Page 26:
"Overall, BNT162b2 elicited HIGHER antigen-binding and neutralizing responses in YOUNGER participants (Figure 6) than in older participants (Figure 7). The BOOST effect AFTER receiving Dose 2 was MOST PRONOUNCED at the 30 μg dose level for OLDER participants."
"Overall, BNT162b2 elicited HIGHER antigen-binding and neutralizing responses in YOUNGER participants (Figure 6) than in older participants (Figure 7). The BOOST effect AFTER receiving Dose 2 was MOST PRONOUNCED at the 30 μg dose level for OLDER participants."
On Page 81, you can see the SI-binding igG (antibodies) measurements for different doses across the 18-55 age groups.
For the 30 µg dose (which is what is being used), the value increases in the first 7 days after the 2nd dose by 722%, from 1264.8 to 9136.4
For the 30 µg dose (which is what is being used), the value increases in the first 7 days after the 2nd dose by 722%, from 1264.8 to 9136.4

On Page 82, you can see the equivalent chart for the 65-85 age group.
First key point is that the antibody levels at Day 21 (after 1st dose) is 283.2 - this represents only 22.4% of the equivalent value for the 18-55 age group.
That's not overly reassuring. 😳
First key point is that the antibody levels at Day 21 (after 1st dose) is 283.2 - this represents only 22.4% of the equivalent value for the 18-55 age group.
That's not overly reassuring. 😳

However, for this age group, there is a massive spike in antibody levels after the 2nd dose is given.
For the 30 µg dose, this value increases in the first 7 days by 2665%, from 283.2 to 7547.9
This represents 82.6% of the equivalent figure for 18-55s.
For the 30 µg dose, this value increases in the first 7 days by 2665%, from 283.2 to 7547.9
This represents 82.6% of the equivalent figure for 18-55s.
So, we can see from this data that there is a much lower initial immune response in the older age group (which is typical for older people).
In the 7 days after the 2nd dose has been given, while still lower than for the 18-55 group, the levels are at least in the same ballpark.
In the 7 days after the 2nd dose has been given, while still lower than for the 18-55 group, the levels are at least in the same ballpark.
An important point to note is that we currently have no idea about what level of antibodies is actually required to mount an effective defence against Covid-19.
For Pfizer, we only know that if we follow the procedure used in the Phase 3 clinical trial, it has 95% efficacy.
For Pfizer, we only know that if we follow the procedure used in the Phase 3 clinical trial, it has 95% efficacy.
Could it achieve 95% efficacy with much lower levels of antibodies?
Yes, absolutely.
It's a BIG gamble.
We have literally no idea where that level could be, or how badly efficacy could be affected as we get close to it, or even fall below that threshold.
Yes, absolutely.
It's a BIG gamble.
We have literally no idea where that level could be, or how badly efficacy could be affected as we get close to it, or even fall below that threshold.
The JCVI said (see Appendix A):
rcgp.org.uk/-/media/Files/…:
"A reasonable interval to use for post first dose VE (Vaccine Efficacy) would therefore be from >14 days (after the 1st dose) to the time of the second dose (scheduled 21 days after the 1st dose) or to...
rcgp.org.uk/-/media/Files/…:
"A reasonable interval to use for post first dose VE (Vaccine Efficacy) would therefore be from >14 days (after the 1st dose) to the time of the second dose (scheduled 21 days after the 1st dose) or to...
...7 days after the second dose base on the ASSUMPTION THE SECOND DOSE WOULD NOT HAVE INDUCED A RESPONSE IN THIS INTERVAL. Unfortunately, this analysis is not presented in the paper."
Unfortunately? Heh! 🤣
Pfizer knew it to be a fiction.
That's why it ISN'T "presented". 🙄
Unfortunately? Heh! 🤣
Pfizer knew it to be a fiction.
That's why it ISN'T "presented". 🙄
Looking at the spike in antibody levels in the 1st 7 days after the 2nd dose, the JCVI assume this is entirely due to the 1st dose.
That (viewed on this data alone) is certainly a hypothesis one could make, amongst others.
One piece of data is a vital clue that disproves this.
That (viewed on this data alone) is certainly a hypothesis one could make, amongst others.
One piece of data is a vital clue that disproves this.
The vaccine which is actually being used by Pfizer is called BNT162b2 (one can only assume the Marketing department were having something of an 'off day').🤷🏻♂️
Covid Crusher would certainly be SLIGHTLY catchier. 😜
Covid Crusher would certainly be SLIGHTLY catchier. 😜
However, Pfizer first carried out Phase 1/2 trials (designed to check safety and work out the correct dose), with another vaccine candidate called (you guessed it) BNT162b1.
That paper was published in Nature.
nature.com/articles/s4158…
That paper was published in Nature.
nature.com/articles/s4158…
For this vaccine candidate, they tested three different doses:
10 µg (2 doses, 21 days apart)
30 µg (2 doses, 21 days apart)
100 µg (1 dose only)
This ISN'T the final vaccine that is being used, but it IS a similar mRNA vaccine and we have VERY limited data on mRNA vaccines.
10 µg (2 doses, 21 days apart)
30 µg (2 doses, 21 days apart)
100 µg (1 dose only)
This ISN'T the final vaccine that is being used, but it IS a similar mRNA vaccine and we have VERY limited data on mRNA vaccines.
If the JCVI assumption that the 2nd dose plays no role in the first 7 days after it is given is correct, one would predict that we would see similar spikes in antibody levels for all three doses of the BNT162b1 vaccine candidate, during that period.
It's that simple.
It's that simple.
What we actually see is:
The 10 ug dose increases by 900% in the 7 days after the 2nd dose is given.
The 30 ug dose increases by 1800% in the 7 days after the 2nd dose is given.
The 100 ug dose DECREASES by 29% in the 7 days when NO 2nd dose is given.
The 10 ug dose increases by 900% in the 7 days after the 2nd dose is given.
The 30 ug dose increases by 1800% in the 7 days after the 2nd dose is given.
The 100 ug dose DECREASES by 29% in the 7 days when NO 2nd dose is given.

Accordingly, one could best describe the assumption, on which the JCVI have chosen to base a mass vaccination strategy as "significantly flawed" (if one is being polite), or "complete bollocks" (if one is being somewhat less polite).
Having found the Nature paper several weeks ago, I was clear that the UK was taking a gamble on its vaccination strategy, with regards to the Pfizer vaccine.
This new data, clearly shows that this is an even LARGER gamble, for the most vulnerable age groups in the UK.
This new data, clearly shows that this is an even LARGER gamble, for the most vulnerable age groups in the UK.
What is contemptible is the public messaging associated with this gamble, presents it as being anything but a gamble.
1 Pfizer dose = 90% protection
2 Pfizer doses = 95% protection
Yeah, IF you follow the clinical trial procedure.
Deviate and it's a hopeful Hail Mary pass.
1 Pfizer dose = 90% protection
2 Pfizer doses = 95% protection
Yeah, IF you follow the clinical trial procedure.
Deviate and it's a hopeful Hail Mary pass.
As patients, we deserve better. If we are invited to embrace risk, there is a duty to tell us that we are facing increased risk.
As citizens, we deserve better.
How do electors evaluate the performance/judgement of our political leaders, if we are being kept in the dark?
As citizens, we deserve better.
How do electors evaluate the performance/judgement of our political leaders, if we are being kept in the dark?
Let's say one's spouse or partner visits a casino once a week and gambles their entire salary on a single turn of a roulette wheel, always betting on red.
They don't tell you that they are doing this.
If red keeps coming up, you may be none the wiser.
They don't tell you that they are doing this.
If red keeps coming up, you may be none the wiser.
However, if you remain unaware of their habitual willingness to embrace risks (which could affect you VERY adversely), you are hardly in a position to make an informed judgement on how well your relationship is going.
This is a matter of public health.
It is also a matter of public trust.
Consent of the governed, comes with a reasonable expectation that citizens will be told the truth, (especially in matters of life and death), in a way that allows them to give INFORMED consent.
It is also a matter of public trust.
Consent of the governed, comes with a reasonable expectation that citizens will be told the truth, (especially in matters of life and death), in a way that allows them to give INFORMED consent.
• • •
Missing some Tweet in this thread? You can try to
force a refresh