I am super excited to share our latest work!!
We developed a human model of Leigh syndrome with brain organoids. Thread below.
Led by @GizCicada with @N_Rajewsky
@MDC_Berlin, @HHU_de, @MedHHU, @StemCellsNRW, @SpringerNature, @NatureComms
Full text: rdcu.be/chxc4
We developed a human model of Leigh syndrome with brain organoids. Thread below.
Led by @GizCicada with @N_Rajewsky
@MDC_Berlin, @HHU_de, @MedHHU, @StemCellsNRW, @SpringerNature, @NatureComms
Full text: rdcu.be/chxc4
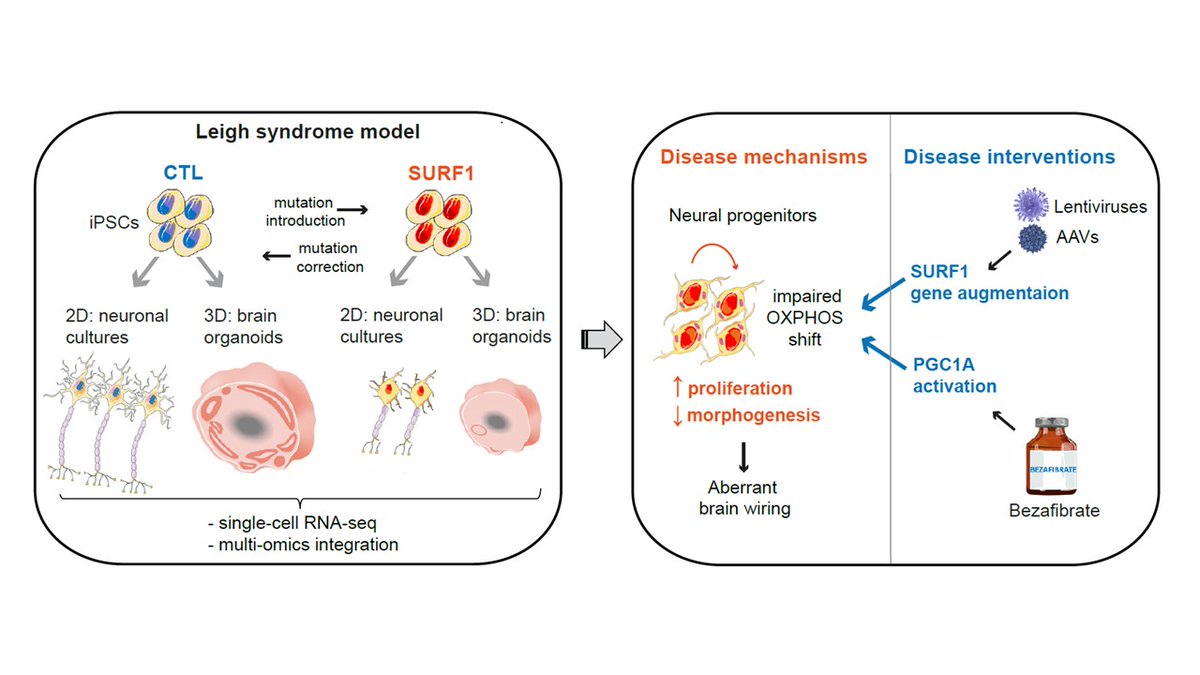
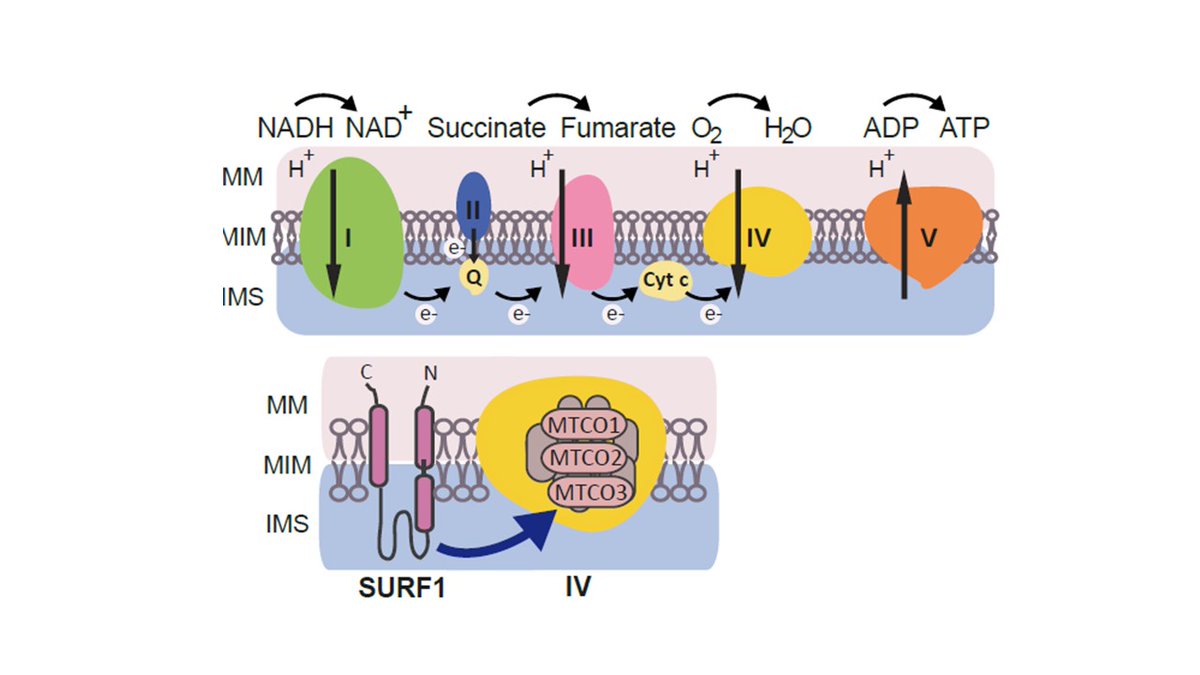
Leigh syndrome is a #RareDisease caused by #mitochondria defects, causing neurodevelpmental impairment and early death. No treatments are available . SURF1 mutations are a main cause but animal models do not recapitulate clinical features.
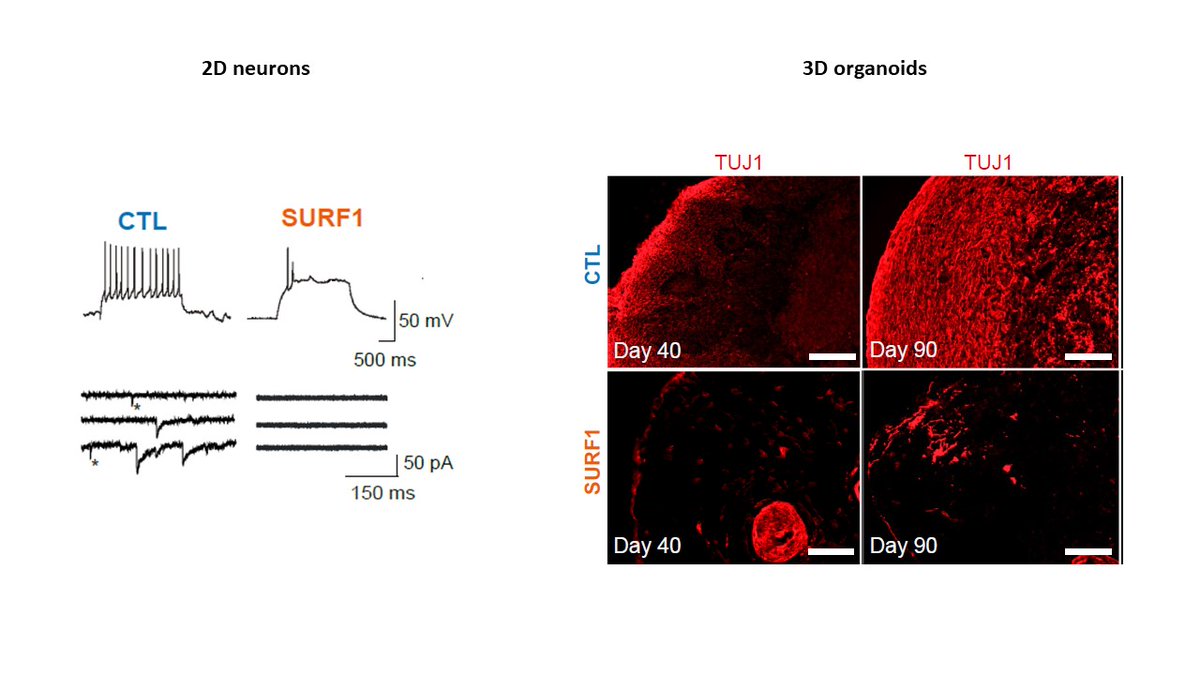
We used patient-specific iPSCs and CRISPR/Cas9 to generate a human model using 2D neurons and 3D brain organoids.
SURF1 is an assembly factor of mitochondrial complex IV.
Possibly because of species-specific differences to complex assembly, the animal models do not fully recapitulate the neurological defects of the patients
Possibly because of species-specific differences to complex assembly, the animal models do not fully recapitulate the neurological defects of the patients
Using scRNAseq of 2D neurons and 3D organoids, we identified a defect in the cell cycle and proliferation of progenitor cells 
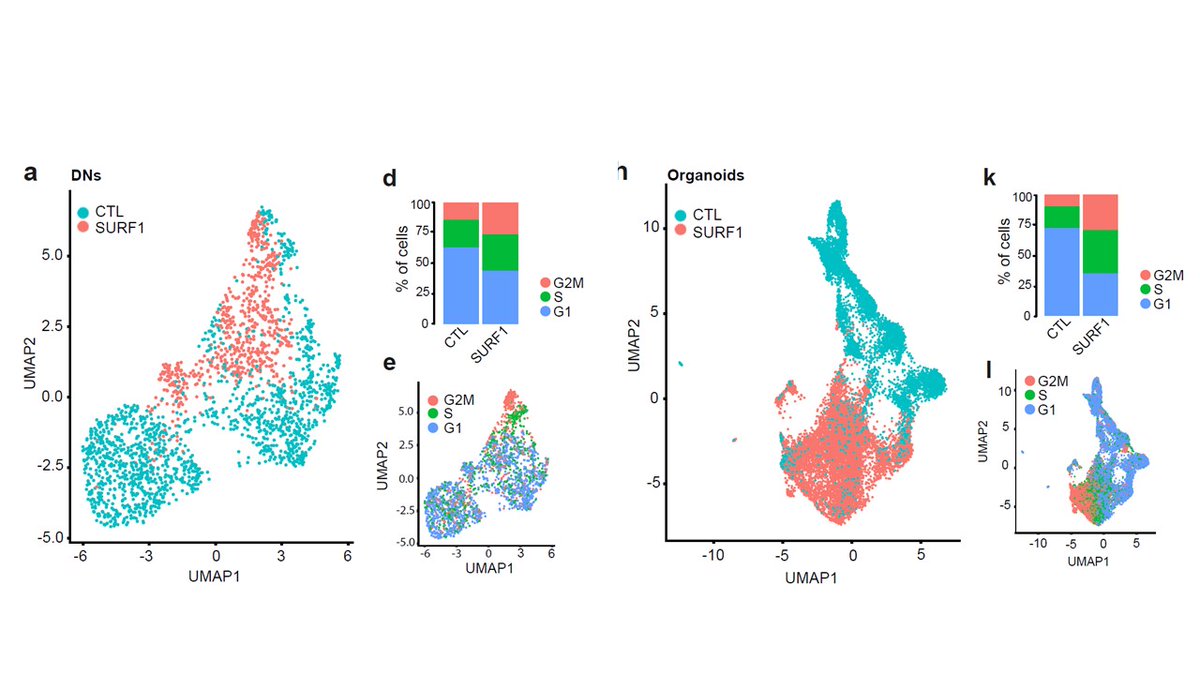
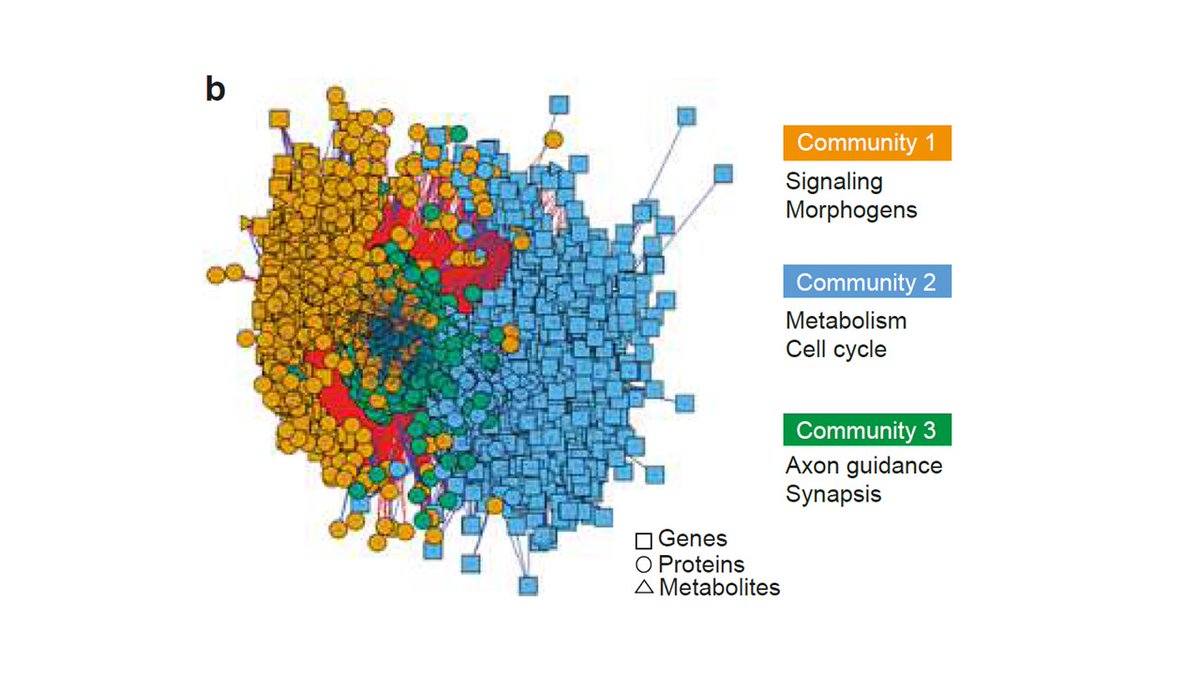
We then integrated transcriptomics, proteomics, and metabolomics, and indentified defective signature in neuronal morphogenesis 
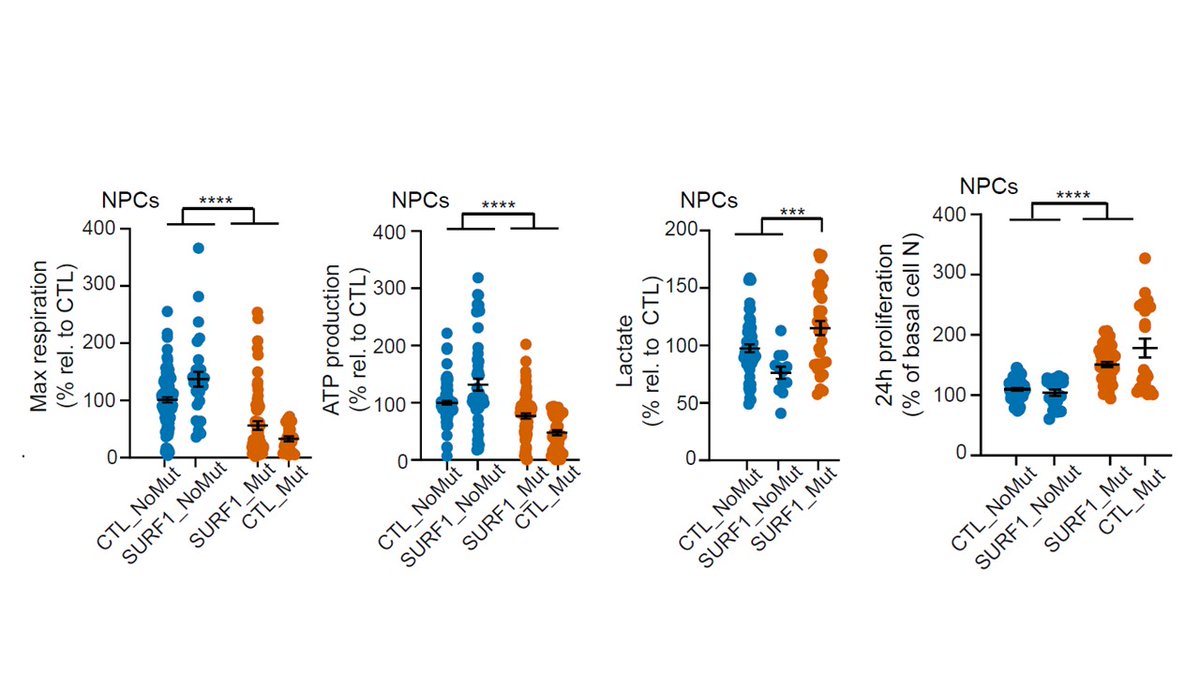
Basically neurons failed to properly mature because neural progenitor cells (NPCs) were not capable of swithing to mitochondrial metabolism and retained a proliferative and glycolytic phenotype 
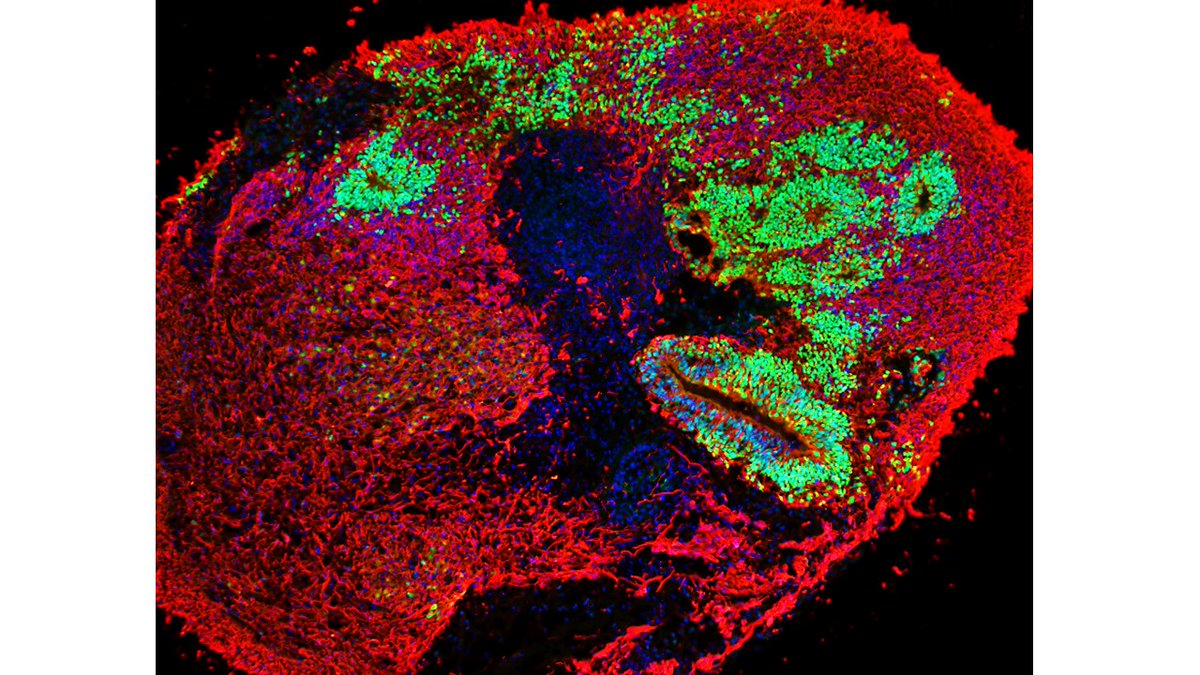
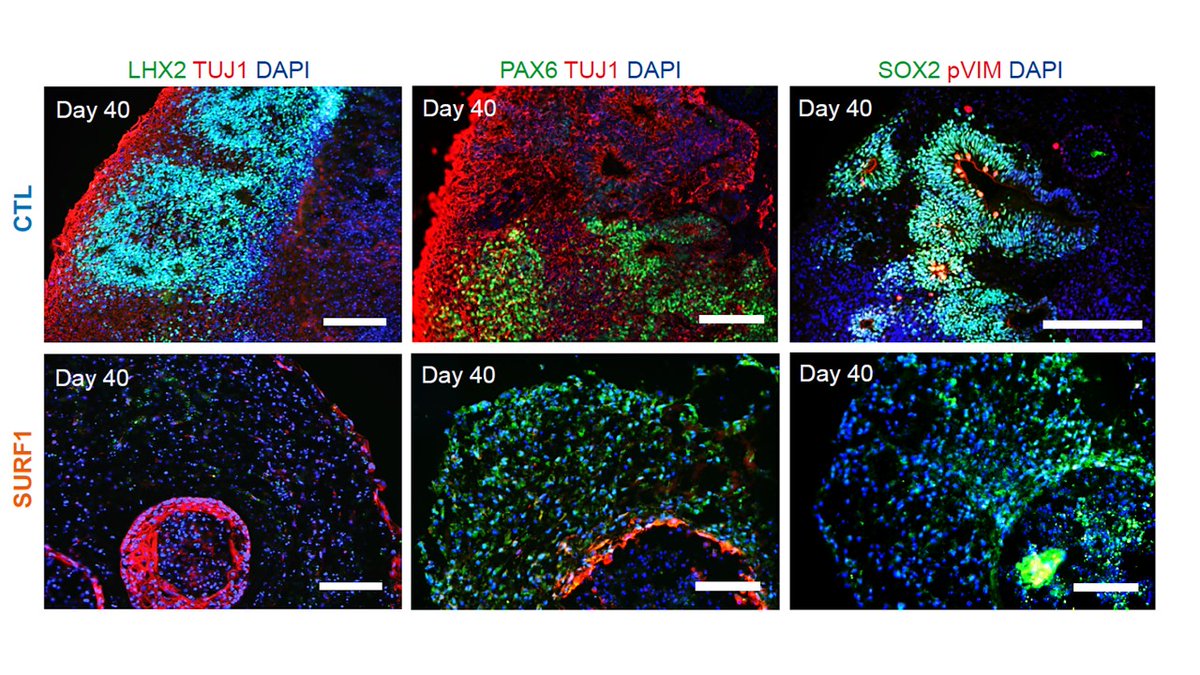
In fact, in brain organoids carrying SURF1 mutations neural progenitors show abnormal architecture and expression. 
This NPC phenotype gave us an entry point for searching for potential therapies.
We did not show beneficial effects for hypoxia or antioxidants.
But overexpression of PGC1A using bezafibrate (BZ) was able to promote the metabolic shift of NPCs and early morphogenesis.
We did not show beneficial effects for hypoxia or antioxidants.
But overexpression of PGC1A using bezafibrate (BZ) was able to promote the metabolic shift of NPCs and early morphogenesis.
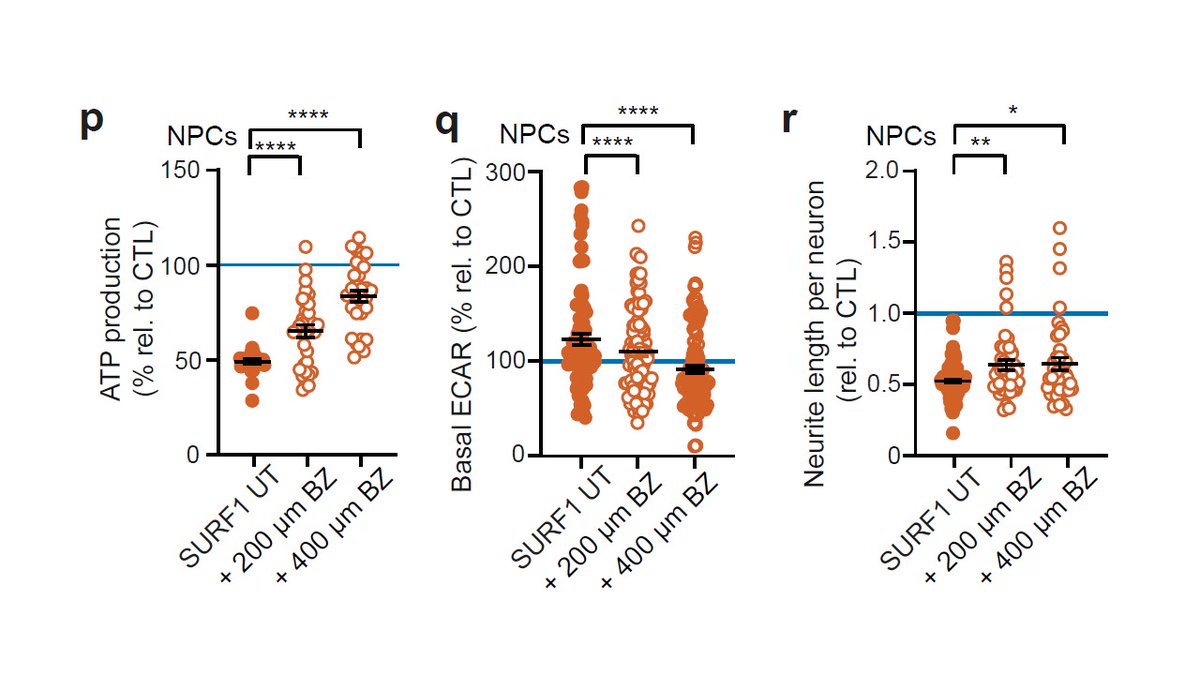
Altogether, Leigh syndrome impacts the metabolic programming of neural progenitors, leading to disrupted neurodevelopment.
Strategies able to improve the metabolic shift of NPCs could then have the potential to be used as interventional therapies.
Strategies able to improve the metabolic shift of NPCs could then have the potential to be used as interventional therapies.
This was huge efforts from several people that I deeply thank.
I am grateful for funding from @dfg_public, @BMBF_Bund, @MDC, @UniklinikDUS, @berlinnovation.
I am grateful for funding from @dfg_public, @BMBF_Bund, @MDC, @UniklinikDUS, @berlinnovation.
We had many setbacks when we were trying to publish this.
Several high-profile journals said it was not of sufficient broad interest (rare diseases may not attract high citations).
I hope that they are wrong and that scientists and patient families will find it interesting!
Several high-profile journals said it was not of sufficient broad interest (rare diseases may not attract high citations).
I hope that they are wrong and that scientists and patient families will find it interesting!
Also thanks to @UMDF for additional support!
• • •
Missing some Tweet in this thread? You can try to
force a refresh