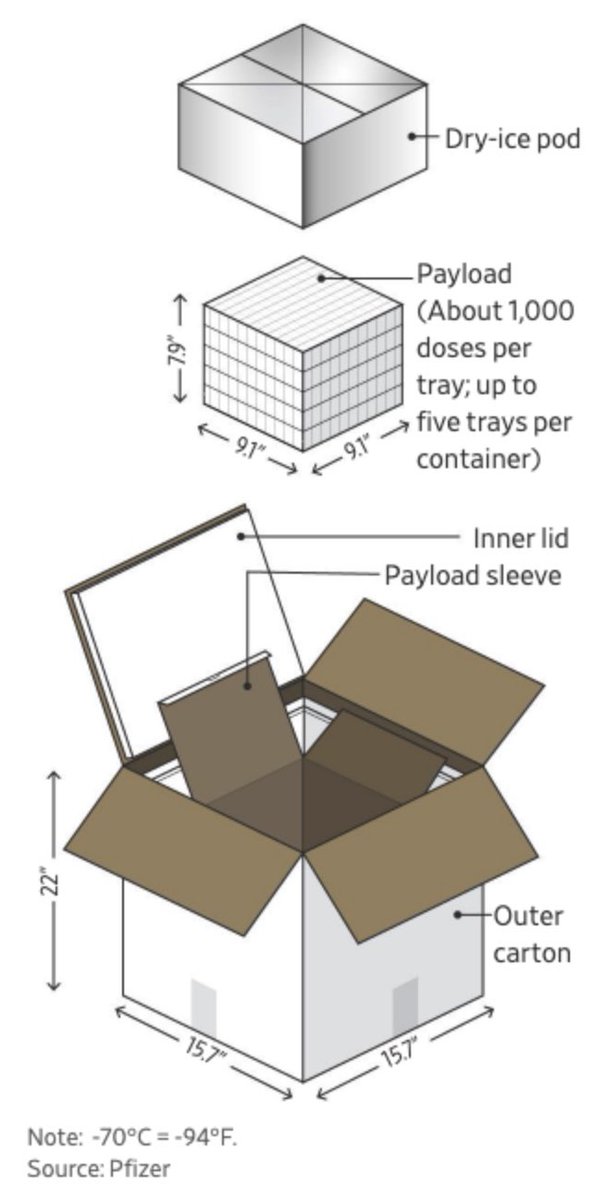
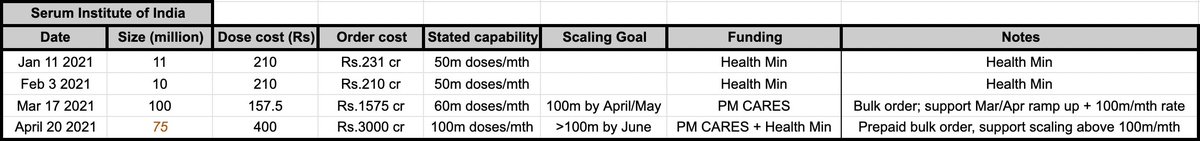
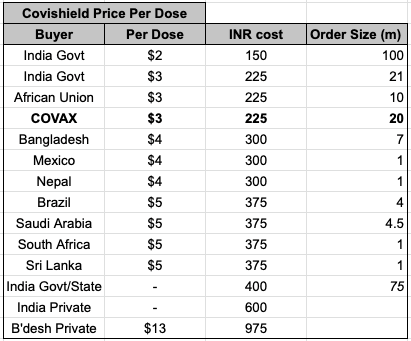
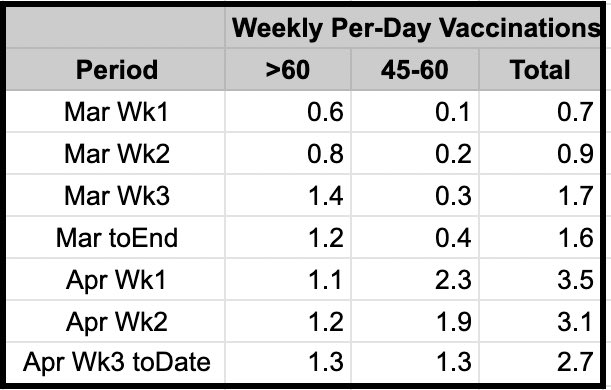
Great thread that takes apart WSJ's predictable opposition to the TRIPS waiver that would help expand COVID vaccine production. The @WSJ is stridently focused on avoiding any attempt to learn from history.
@prasannavishy @c_aashish @amargov @centerofright
@prasannavishy @c_aashish @amargov @centerofright
https://twitter.com/realtahiramin/status/1331010833586020353
The TRIPS waiver was first mooted in October 2020 by India and South Africa:
docs.wto.org/dol2fe/Pages/S…
1/
docs.wto.org/dol2fe/Pages/S…
1/
A wider document was circulated in January 2021 with the signatories list expanding dramatically:
docs.wto.org/dol2fe/Pages/F…
2/
docs.wto.org/dol2fe/Pages/F…
2/
The October and January efforts both failed. The meetings were closed door events:
lifesciencesipreview.com/news/wto-decli…
3/
lifesciencesipreview.com/news/wto-decli…
3/
The following WTO summary page has details on communications by different countries on this topic, from late Feb 2021:
wto.org/english/news_e…
4/
wto.org/english/news_e…
4/
In the US, the pharma lobby pushed Biden to oppose the waiver in early March, using standard cut and paste arguments dating back decades:
jdsupra.com/legalnews/bio-…
5/
jdsupra.com/legalnews/bio-…
5/
The current effort is the fourth one. It has resulted in lobbying on both sides:
aipla.org/detail/news/20…
reuters.com/business/healt…
7/
aipla.org/detail/news/20…
reuters.com/business/healt…
7/
It's unclear if it will pass. On the positive side, India has not one but potentially at least two unique vaccine IPs (Covaxin and the DNA-based Zycov-D in advanced trials).
8/
8/
This is a departure in one more way: Covaxin is the only inactivated viral vaccine. Previous examples quoted have involved clean room re-engineering of western IP. Covaxin & Zycov-D are the first vaccines using their respective technologies in the world.
9/
9/
However this does not mean India should not support the waiver any longer - there are simply too many people still a long way from accessing vaccines, even if India can independently devise and manufacture what it needs for itself.
10/10
10/10
• • •
Missing some Tweet in this thread? You can try to
force a refresh