Insulin was invented ~100 years ago, and its patent sold for $1.
“Do you know the reason why [insulin is] $1,000 with insurance?” ~@AOC
There is no reason for insulin to cost $21 in Canada for a 10 ml bottle, while it costs a mortgage payment in the US.
“Do you know the reason why [insulin is] $1,000 with insurance?” ~@AOC
There is no reason for insulin to cost $21 in Canada for a 10 ml bottle, while it costs a mortgage payment in the US.
2) atrocious amount of big pharma price gouging on insulin. Absolutely atrocious. Heartbreaking atrocious. Shamefully atrocious.
https://twitter.com/DrEricDing/status/1349405447988588545
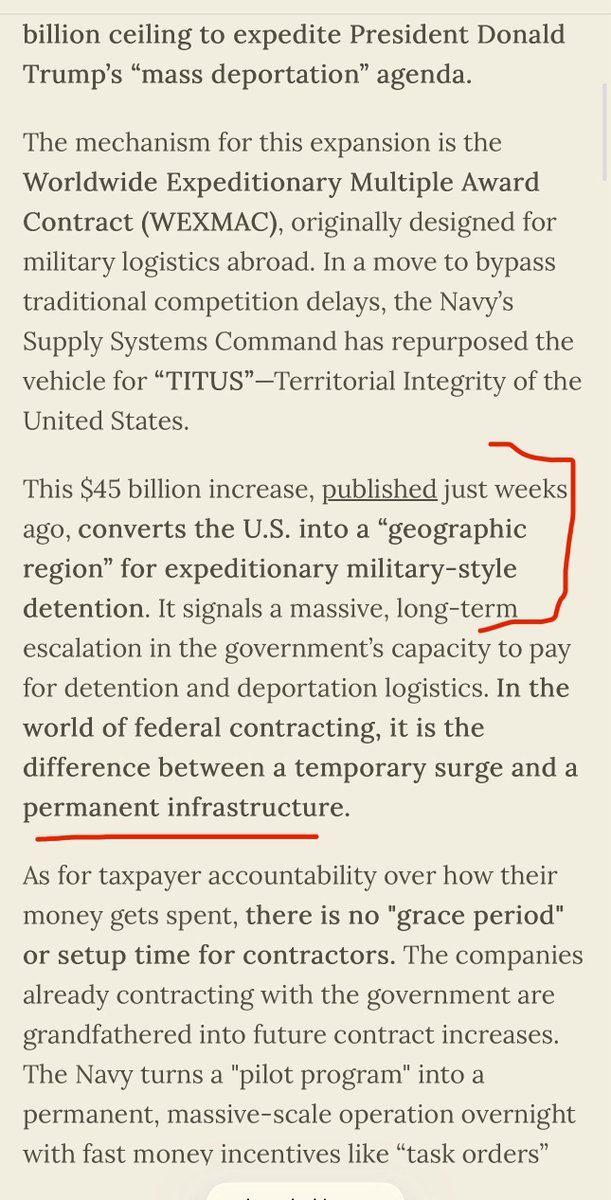
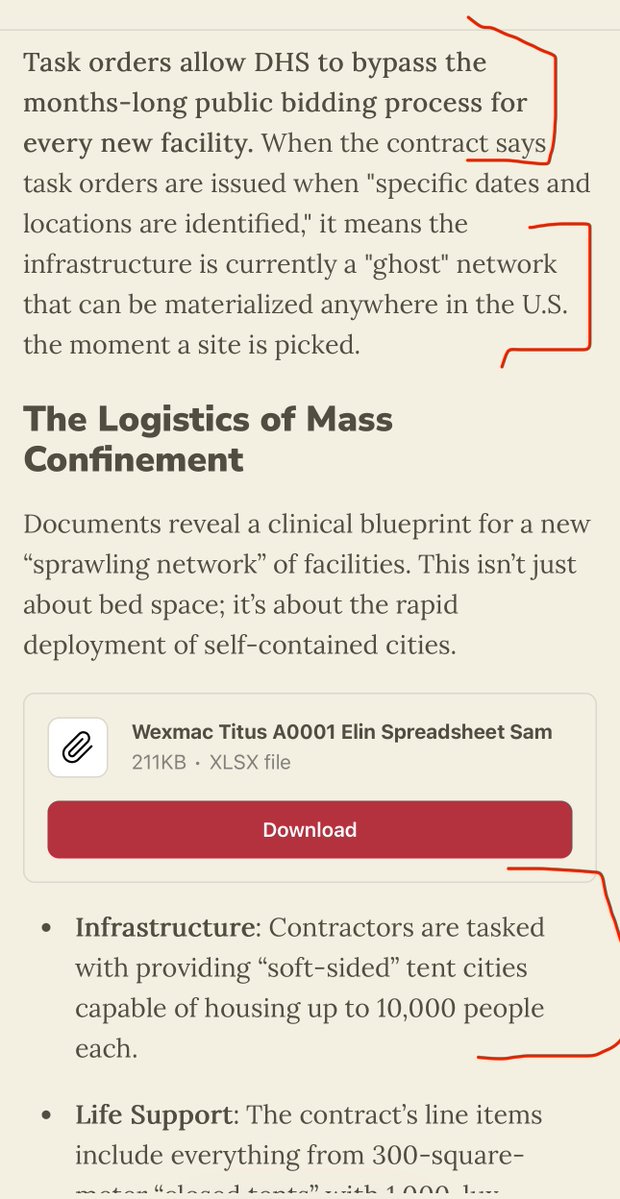
3) Let this sink in:
In 2018:
J&J CEO made $30M
Pfizer CEO made $28M
Merck CEO made $18M
Eli Lilly CEO made $16M
Gilead CEO made $16M
While:
📌Americans spent $535B on Rx drugs
📌1/4 of diabetics rationed insulin to survive
📌500K Americans went bankrupt from medical bills.
In 2018:
J&J CEO made $30M
Pfizer CEO made $28M
Merck CEO made $18M
Eli Lilly CEO made $16M
Gilead CEO made $16M
While:
📌Americans spent $535B on Rx drugs
📌1/4 of diabetics rationed insulin to survive
📌500K Americans went bankrupt from medical bills.
4) Did you know? Medicare, the government health program for those over age 65 (also the nation’s largest buyer of drugs)—is barred from negotiating drug prices. That gives pharma more leverage, and it leads to the kind of price surges of EpiPens, opioid antidotes, & insulin.
5) There are many more reasons for the high cost of insulin. Dr @VincentRK of Mayo Clinic lays out the reasons why. It’s a damn crisis in the US
…rivaling even #COVID19. There I said it!
Really good article…
mayoclinicproceedings.org/article/S0025-…
…rivaling even #COVID19. There I said it!
Really good article…
mayoclinicproceedings.org/article/S0025-…

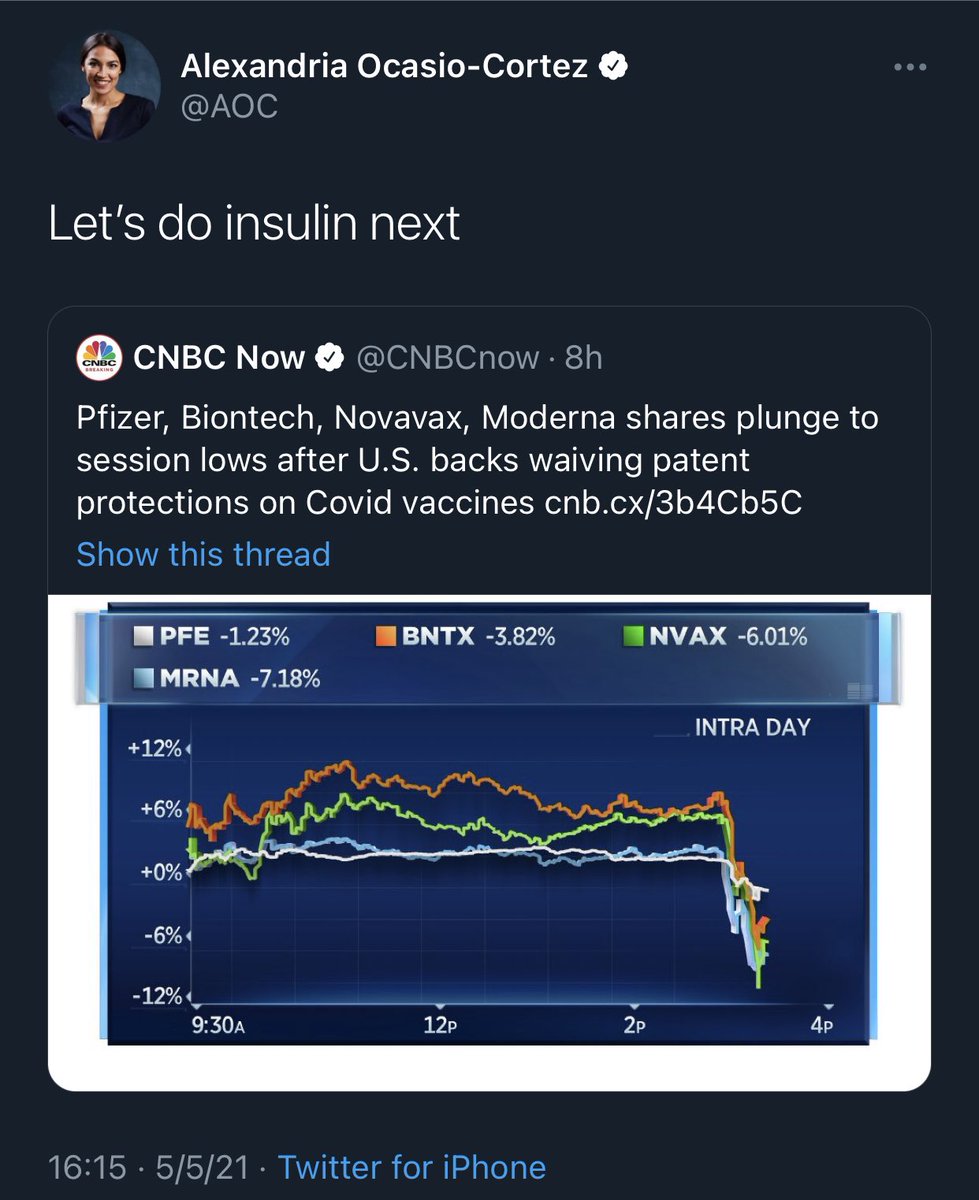
6) Hell yes… waive the patents. Not just for #COVID19, but for insulin too. Diabetes epidemic is just as bad worldwide. 
8) Pharma talks a big game about “free market”—but that doesn’t hold up when they block imports/trade and when medicines are essential “needs” not mere casual “wants” like a new iPhone. You will pass on luxury good, but you can’t pass on that $5000 to save your family.
9) Same for insulin and for Covid vaccines…
Too few companies control too much of the world’s supply. We need the patents waived and knowledge shared to ramp up vaccinations to truly end the pandemic.
Too few companies control too much of the world’s supply. We need the patents waived and knowledge shared to ramp up vaccinations to truly end the pandemic.
https://twitter.com/oksanapyzikucl/status/1390284474693345281
10) Progress… now we need to make Canada 🇨🇦 commit to the #TRIPSwaiver and to make EU and UK commit as well.
https://twitter.com/astroehlein/status/1390040809978880002
11) Not just insulin price gouging, parents of kids with allergies are forced to shell out $600 for EpiPen because it is a matter of life and death. Here is a dark story about Mylan, the EpiPen maker who pharma jacked up the price from a few $ to $600.
12) Mylan didn’t even invent epinephrine, which is simply our human hormone, better known as *adrenaline*, packaged into an 💉 injector (not some wild invention), invented by scientists paid by taxpayers. But big pharma gets to hold parents of kids with allergies hostage.
13) Even worse, Mylan was also recently ***criminally charged*** by Department of Justice for outright fraudulent EpiPen billing, and fined $465 million by DOJ for its corrupt behavior in violating False Claims Act. justice.gov/opa/pr/mylan-a…
14) What we need most of all is #MedicareForAll.
I used to study predictors of universal health care. Key factors of success:
📌Not dictatorship—10x
📌Proportional representation elections—2.8x
📌Less ethnic fractionalization—2x
📌Less wealth inequality sciencedirect.com/science/articl…
I used to study predictors of universal health care. Key factors of success:
📌Not dictatorship—10x
📌Proportional representation elections—2.8x
📌Less ethnic fractionalization—2x
📌Less wealth inequality sciencedirect.com/science/articl…
• • •
Missing some Tweet in this thread? You can try to
force a refresh