
Key takeaways from LOTIS-2 study leading to Loncastuximab approval in R/R DLBCL # Tweetorial #lymsm @ccarlostella @paolocaimiMD
Open-label; single arm; Single agent (important for efficacy data)
No exclusion for pri refrac; double hit NOT excluded (1/10)
thelancet.com/journals/lanon…
Open-label; single arm; Single agent (important for efficacy data)
No exclusion for pri refrac; double hit NOT excluded (1/10)
thelancet.com/journals/lanon…
LOTIS-2 dosing of 150 µg/kg every 3 weeks (Q3W) for 2 doses followed by 75 µg/kg Q3W deduced from large phase 1 study in @BloodJournal (2/10)
pubmed.ncbi.nlm.nih.gov/33211842/
pubmed.ncbi.nlm.nih.gov/33211842/
LOTIS 2; N enrolled = 145
ORR = 70 of 145 (48·3% [95% CI 39·9–56·7]);
35 had complete response and 35 had partial response.
Median DOR 10.3 mons
(3/10)
ORR = 70 of 145 (48·3% [95% CI 39·9–56·7]);
35 had complete response and 35 had partial response.
Median DOR 10.3 mons
(3/10)
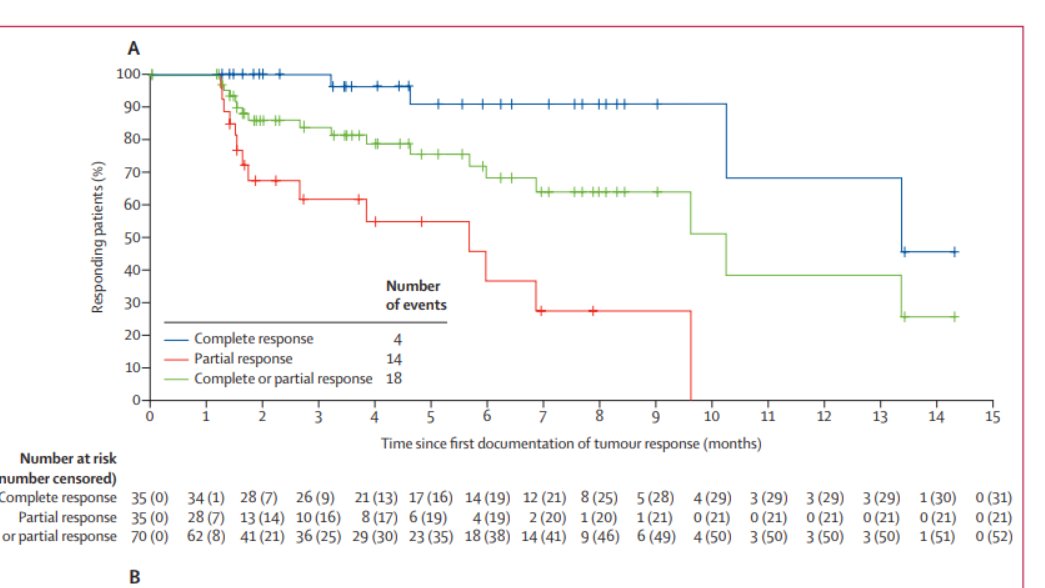
(4/10) Notable toxicities of clinical importance IMO and remedies
Rash/photosensitivity = steroids/hold Rx
⬆️AST/ALT/GGT = Grade 3 hold Rx till resolution
⚠️⚠️⚠️Effusions/Edema = ⚕️Beware; MUST use Dex prophy; treat with steroids. MUST be aware of this!!!
Rash/photosensitivity = steroids/hold Rx
⬆️AST/ALT/GGT = Grade 3 hold Rx till resolution
⚠️⚠️⚠️Effusions/Edema = ⚕️Beware; MUST use Dex prophy; treat with steroids. MUST be aware of this!!!
(5/10) CD19 loss uncommon after Lonca treatment and we have shown that anti CD19 CAR-T treatment after this drug is feasible @BloodJournal by @bickythapa02 @paolocaimiMD ashpublications.org/bloodadvances/…
(6/10) In patients failing anti CD19 CAR-T w/o CD19, Lonca is active. Manuscript ready to be submitted by @paolocaimiMD #cart
(7/10) Key difference from other approved agent studies
Pola/BR = 27% had only 1 prior line; no DHL; no transformed
L-MIND = 50% had only 1 prior line; no primary refractory
Selinexor = no comments🤯
Zynlonta = 2 prior lines required; DHL or transformed included. 👉Excluded bulky
Pola/BR = 27% had only 1 prior line; no DHL; no transformed
L-MIND = 50% had only 1 prior line; no primary refractory
Selinexor = no comments🤯
Zynlonta = 2 prior lines required; DHL or transformed included. 👉Excluded bulky
(8/10) Directions for future development
Lonca combos with other agents
Possible frontline assessment
In CAR failures
Lonca combos with other agents
Possible frontline assessment
In CAR failures
(9/10) Confirmatory phase 3 comparing Lonca vs. R-Gem-Ox launched. IMO single agent head-2-head with a real regimen (unlike BR in DLBCL) @VPrasadMDMPH
clinicaltrials.gov/ct2/show/NCT04…
clinicaltrials.gov/ct2/show/NCT04…
(10/10) finally my COI = Investigator & consultancy for @adctherapeutics
Excellent editorial by the fabulous @kmaddmd thelancet.com/journals/lanon…
Excellent editorial by the fabulous @kmaddmd thelancet.com/journals/lanon…
• • •
Missing some Tweet in this thread? You can try to
force a refresh



