Once again medicines regulations are becoming politically charged in the UK. Late last year it was rapid / hasty (depending in your view) MHRA authorization of COVID vaccines. Now it is about cancer treatments.
Brief thread on regulatory approval and market access.
/1
Brief thread on regulatory approval and market access.
/1
https://twitter.com/SJAMcBride/status/1393461864315633666
For your doctor to prescribe you a medicine, two things must be true (outside a clinical trial)
1. The medicine can legally be sold in the place you live (regulatory marketing authorization)
2. Someone (hopefully by you) is willing to pay for that medicine (reimbursement)
/2
1. The medicine can legally be sold in the place you live (regulatory marketing authorization)
2. Someone (hopefully by you) is willing to pay for that medicine (reimbursement)
/2
The regulators make sure that medicines are only sold if there is strong evidence that the balance of benefit to risk is favourable. Their goal is to stop companies marketing medicines that are unsafe or don't work.
The "label" on a medicine is key.
/3
The "label" on a medicine is key.
/3
A medicine label includes the indication: for what type of patients/illness has the pharma company produced compelling evidence of safety and efficacy.
A new drug typically starts with an indication for one (sometimes small) patient population.
/4
A new drug typically starts with an indication for one (sometimes small) patient population.
/4
But the label indication evolves over time. "Label expansion" is the process of doing further clinical trials to show the med works in other types ilof patients or illness.
So if the label approved by my local regulator includes me, do I get the drug?
Well not necessarily.
/5
So if the label approved by my local regulator includes me, do I get the drug?
Well not necessarily.
/5
Whether you get a medicines also depends on whether the person paying for your care considers it value for money.
This involves the pharma company producing evidence of health economic benefit to justify the price (value based pricing).
Expensive drugs are rationed.
/6
This involves the pharma company producing evidence of health economic benefit to justify the price (value based pricing).
Expensive drugs are rationed.
/6
But you might get a medicine even if your illness isn't on the label. This is called "off label" use and is under the discretion of your doctor. It is influenced by local treatment guidelines and oubkused papers.
Drug companies can't market a drug for off label use.
/7
Drug companies can't market a drug for off label use.
/7
If your regulator has authorized a med for one indication, you might get it for that, or something else. But you might not get it for either depending on reimbursement and local clinical practice.
But if it isn't authorized for anything, it can't be sold and you can't get it.
/8
But if it isn't authorized for anything, it can't be sold and you can't get it.
/8
To give a personal example, nearly 15 years ago I had a type if cancer (my twitter handle) and a new anti body treatment was authorized for a later stage of the same cancer. But I got that treatment off label from a London hospital. It was later indicated for my disease.
/9
/9
So back to NI and it's unusual regulatory position.
The controversy over the cancer treatment Osimertinib which how has a different label from EMA (hence NI) to MHRA (GB).
This can be dealt with through UK wide treatment guidelines for off label use
/10
The controversy over the cancer treatment Osimertinib which how has a different label from EMA (hence NI) to MHRA (GB).
This can be dealt with through UK wide treatment guidelines for off label use
/10
https://twitter.com/hhesterm/status/1393249202361282561?s=20
The bigger problem will come when either EMA or MHRA authorizes an entirely new medicine before the other. Off label use then isn't possible in the jurisdiction with no auth.
The industry concensus has typically been that pharma companies will prioritize EMA over MHRA.
/11
The industry concensus has typically been that pharma companies will prioritize EMA over MHRA.
/11
But MHRA is trying to establish a reputation for rapid review to mitigate this.
Time will tell if they works out.
The politics in NI will however be difficult as EU and UK diverge over medicine (and medical device) regulation.
/12
Time will tell if they works out.
The politics in NI will however be difficult as EU and UK diverge over medicine (and medical device) regulation.
/12
To switch topic slightly... The related field of medical device regulation is also likely to be a significant source of UK/EU divergence and hence NI/GB stresses.
Medicine regulation in Europe is mature and well respected by industry. MHRA has adopted the same regs.
/13
Medicine regulation in Europe is mature and well respected by industry. MHRA has adopted the same regs.
/13
EMA, like MHRA has learned from COVID and will be more agile in review moving forward and so speed of review is unlikely to be very different.
Medical devices regulation in Europe, in contrast, is in transition. Some would say in crisis.
Med devices isn't EMA's job.
/14
Medical devices regulation in Europe, in contrast, is in transition. Some would say in crisis.
Med devices isn't EMA's job.
/14
Medical device regulation in Europe has no central agency. The EU sets device rules and national competent authorities supervise notified bodies who review technical files for CE marking of devices.
/15
/15
The new medical device regulation requires much more clinical evidence that devices work. Especially devices containing complicated software. But expertise in AI and novel sensors is in short supply and spread thin. Many in industry are frustrated by the MDR implementation.
/16
/16
Th FDA is centralised and has its act better together. Many European companies developing innovative devices now go for FDA clearance or authorization prior to CE marking.
The UK MHRA theredore has more space to do things differently in devices then medicines.
/17
The UK MHRA theredore has more space to do things differently in devices then medicines.
/17
So what does this mean for NI if novel medical devices eg: including AI get approved in UK before CE marking?
Well it is complicated.
But as this poster presented at the RAPS conference last week shows that NI ought to get best of both worlds.
/18

Well it is complicated.
But as this poster presented at the RAPS conference last week shows that NI ought to get best of both worlds.
/18

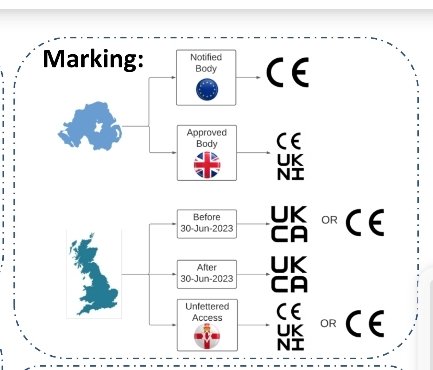
That poster was presented by @sebclerkin at RAPS Euro Convergence meeting. He may be able to say more. 

• • •
Missing some Tweet in this thread? You can try to
force a refresh