Now to talk about Prussian blue analogues! To begin I have to tell the story behind them because it is one of my favourite pieces of chemical history. It is a tale of alchemists, theologians, famous paintings and about 200 years of wondering what Prussian blue was.
It all began in Berlin in 1704 with an enterprising dye maker by the name of Heinrich Diesbach. He was most interested in producing a red dye by the name of Cochineal red lake. The ingredients were iron sulphate, potash and crushed up beetles #alchemy 

But Diesbach was running low on potash. Enter the scene one Johann Conrad Dippel. Master theologian, physician, alchemist. Dippel was captured by the allure of alchemy and like any good alchemist began his attempts at transmuting gold. 

This failed, but it did not deter him from making a miraculous "animal oil" and releasing it upon an unsuspecting public. The concoction, distilled from the ground up remains of animals, was so foul it was believed it must be good for you (like any good medicine right?)
What does this have to do with a dye maker? Diesbach is said to have been preparing his dyes in Dippels laboratories. His potash shortage was solved by buying from Dippel. Now, Dippel was not a great alchemist and a terrible chemist so of course the potash was contaminated
And so, unsuspectingly using the potash contaminated with animal remains Diesbach tried to produce his red dye. He did not get a red dye. He produced a very blue powder instead. 

Keep in mind that obtaining vibrant blue colours for paint or clothing in these times was not easy, and not cheap. Diesbach knew this and quickly attempted to recreate the recipe and sell his new blue pigment to the world leading to several famous paintings. 



The original recipe called for dried ox blood as a key ingredient. Of course, they can't have known what was really going on at the time because the discovery of several key elements had not yet happened (like nitrogen in 1772). So what did they really make?
The key ingredients are iron and cyanide. The cyanide, which originated from various alkyl cyanides in the animal remains, dramatically changed the reaction leading to what is now known as Prussian blue. But it took a few hundred years to figure this out rdcu.be/cm3vx 

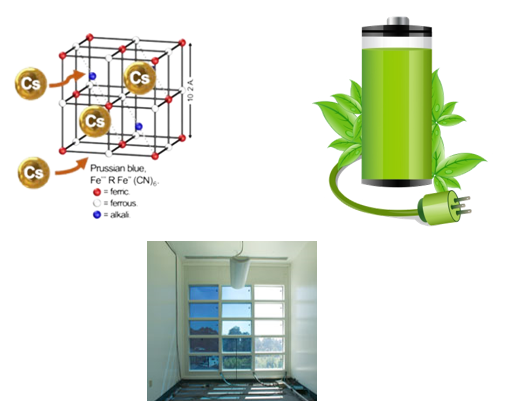
Finally, using X-ray diffraction, it was determined that Prussian blue was a coordination framework compound consisting of iron centers bridged together by cyanide ligands. Each iron can be coordinated by six cyanides giving the porous cubic structure. 

This was just the beginning. Prussian blue is one member of a family of compounds known as Prussian blue analogues. They share the same coordination environment of CN bridges, but the transition metal can change and the pores in the structure can host many different species.
Many different compositions leads to a variety of properties and applications. Prussian blue analogues are more than just pretty colours. They can be used for treating Cesium and thallium poisoning, CO2 capture, in electrochromic windows and of course, in batteries! (and more) 
For more detail on the history of Prussian blue, I recommend the work by Alexander Kraft, who has written a few times on the history of the pigment (acshist.scs.illinois.edu/bulletin_open_…) and also a nice perspective by Andreas Ludi: pubs.acs.org/doi/10.1021/ed…
• • •
Missing some Tweet in this thread? You can try to
force a refresh









