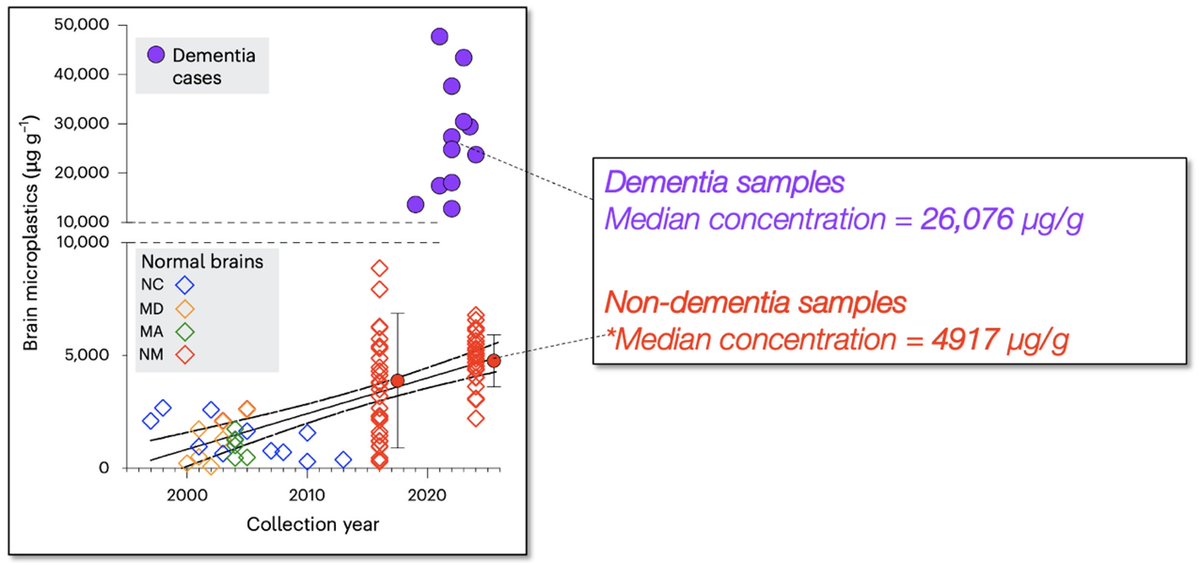
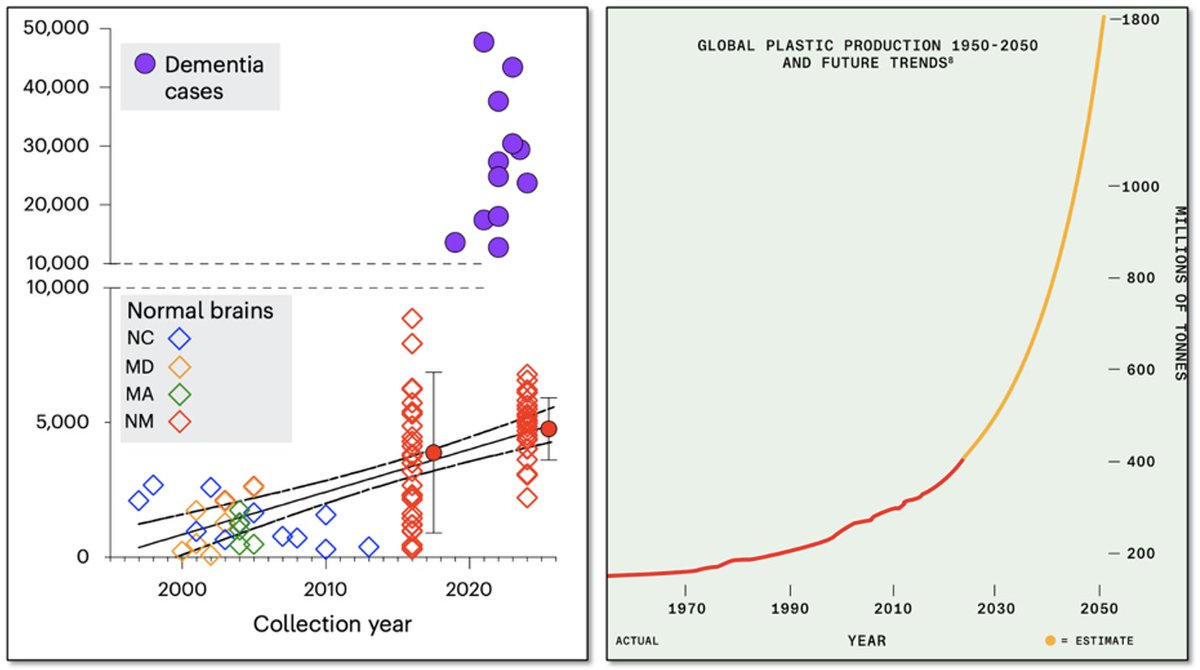
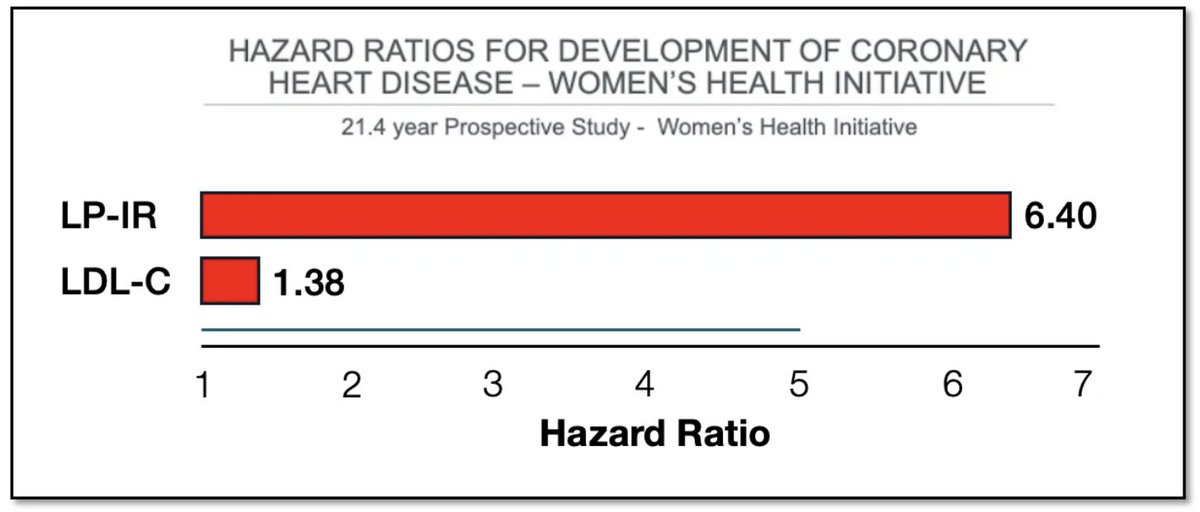
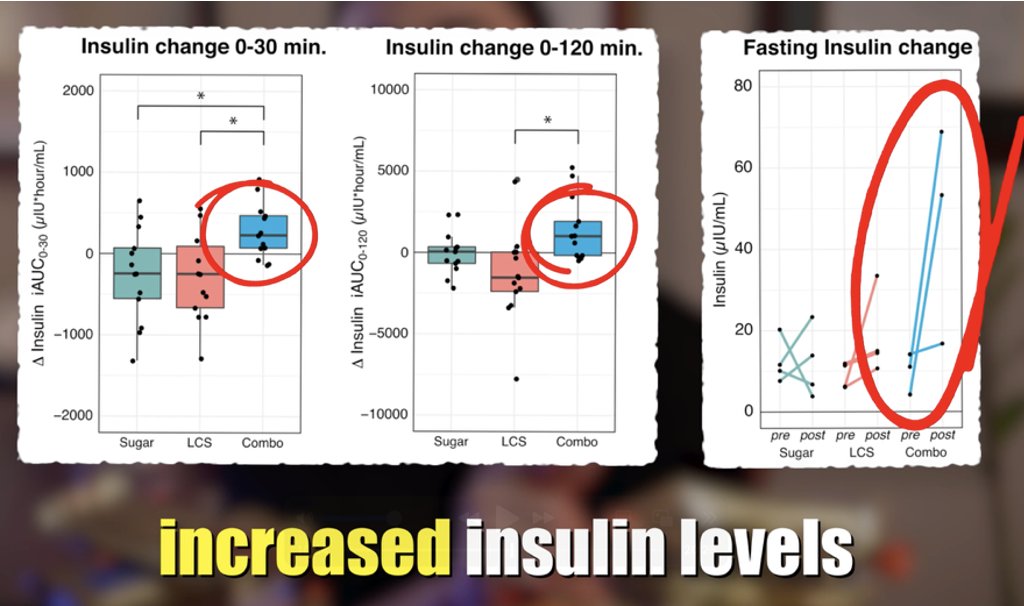
The #ketone body, acetoacetate (AcAc) regulates lipid metabolism through receptor GPR43
pnas.org/content/116/47…
Cool study .@DaveKeto and I were discussing...
AcAc binds to the GPR43/FFAR2 receptor to promote Lipoprotein Lipase activity and help burn fat.
Some more details...
pnas.org/content/116/47…
Cool study .@DaveKeto and I were discussing...
AcAc binds to the GPR43/FFAR2 receptor to promote Lipoprotein Lipase activity and help burn fat.
Some more details...
2/ Short-chain fatty acids (SCFAs) in the gut r known to modulate energy homeostatis. Butyrate, acetate, proprionate all have recptors. The acetate receptor is GPR43.
The ketone BhB is all well studied as a signaling molecule, and binds HCAR2 etc., but AcAc is less well studied.
The ketone BhB is all well studied as a signaling molecule, and binds HCAR2 etc., but AcAc is less well studied.
3/ This paper provides good evidence that, during fasting and ketogenic conditions, its AcAc that helps promote fat burning (lypolysis) throughout the body (except in the gut, more on that in a bit). Again, AcAc binds GPR43 and promotes Lipoprotein lipase (LPL activity)...
4/ The researchers were able to demonstrate this phenomenon using mice that didn't express GPR43, this lead to decreased LPL in their fat cells, elevated Trig levels, decrease energy expenditure, and less weight loss... 



5/ Interestingly, they also found that the AcAc-->GRP43 --> LPL signaling axis, while increasing LPL expression, decreased ANGPLT4 expression - with ANGPLT4 being a protein that inhibits LPL activity. The reason I mean to highlight this is...
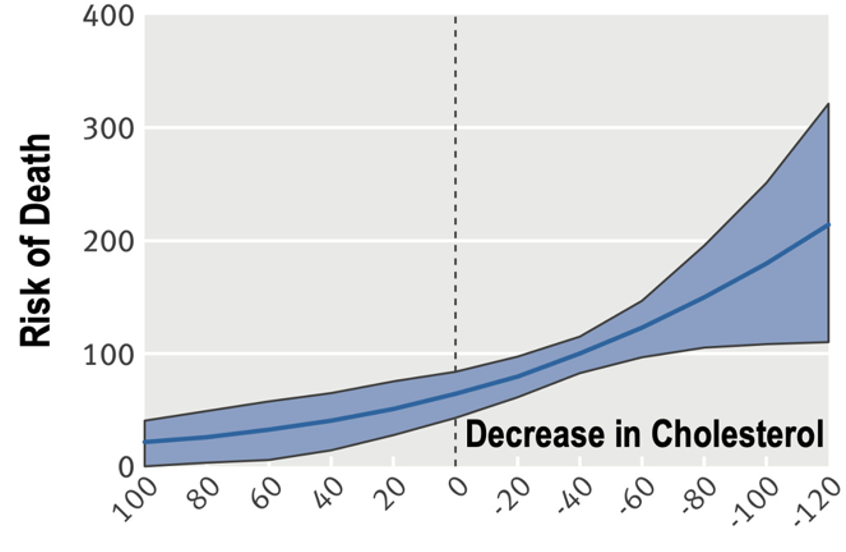
6/ ... is loss of function in LPL and gain of function in ANGPLT4 are each linked to decreased turnover of Trig-rich lipoproteins that are now the subject of scrutinity in terms of development of CVD....
nature.com/articles/s4158…
nature.com/articles/s4158…
7/ They also observed GPR43 signaling was important to regulate energy homeostasisunder ketogenic conditions. GPR43-/- mice actually gained weight (body fat) when fed a ketogenic diet... 

8/ But while fasting and #keto increase AcAc to increase GPR43-mediated LPL activity in fat cells around the body, fasting also decreases acetate production in the gut (of course, because you're not eating). In the gut, acetate remains the main GPR43 ligand. Therefore...
9/ The AcAc / acetate-->GPR43-->LPL axis actualy helps w the choreography of energy homeostasis when fasting
Fat burning is upregulated around the body to supply energy, while LPL activity is decreased in gut to prevent wasting energy on the digetive track
Pretty logical to me
Fat burning is upregulated around the body to supply energy, while LPL activity is decreased in gut to prevent wasting energy on the digetive track
Pretty logical to me
10/ I just thought this was a cool one because we don't often talk about acetoacetate signaling. Also LPL dysfunction may be a common feature in metabolic diseases leading to lipid abnormalities. See where I'm going with this train of thought...
Cool stuff :).
Cool stuff :).
• • •
Missing some Tweet in this thread? You can try to
force a refresh