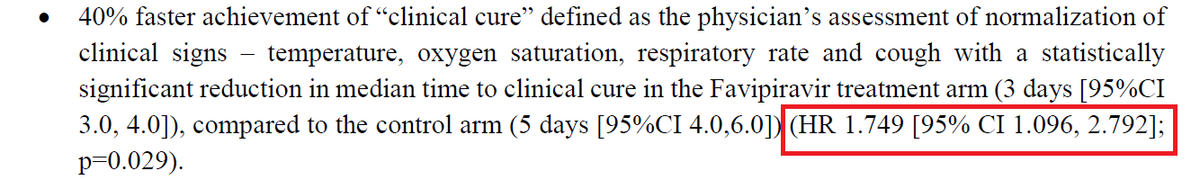1/n
My 7-slide summary of the @NEJM 7 July study on safety and effectivity of #COVID19Vaccination in adolescents
Q What was the research question?
Is Covid vaccine safe and effective in adolescents aged 12-15 years of age?
My 7-slide summary of the @NEJM 7 July study on safety and effectivity of #COVID19Vaccination in adolescents
Q What was the research question?
Is Covid vaccine safe and effective in adolescents aged 12-15 years of age?

2/n
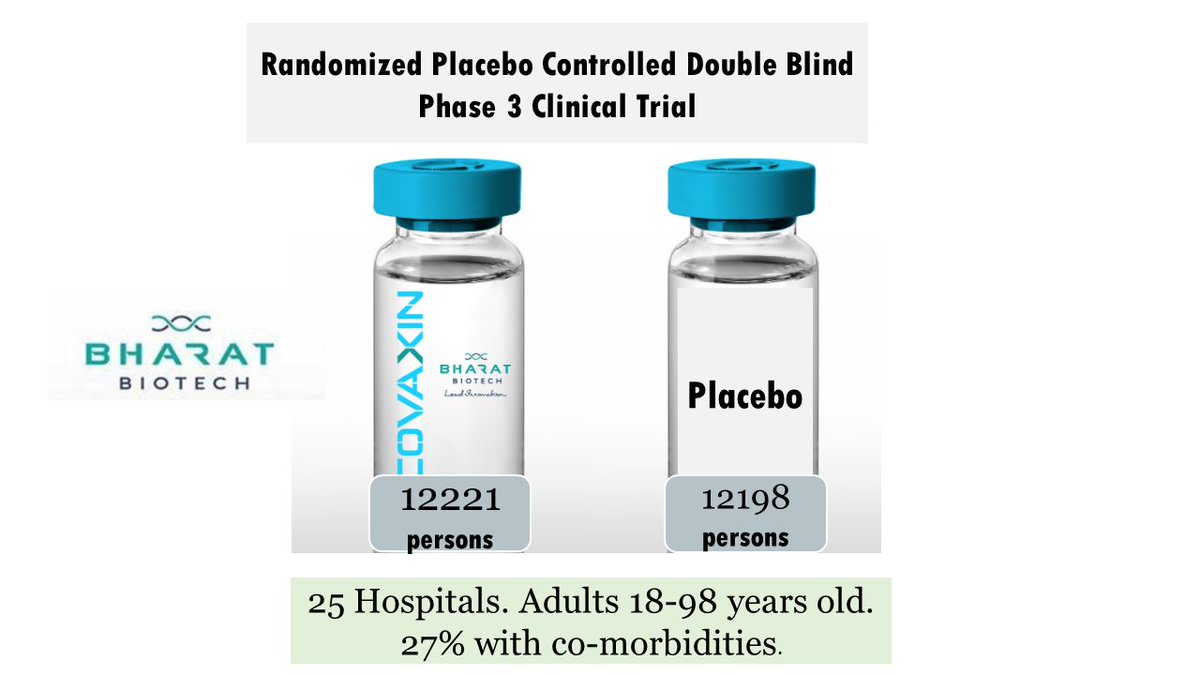
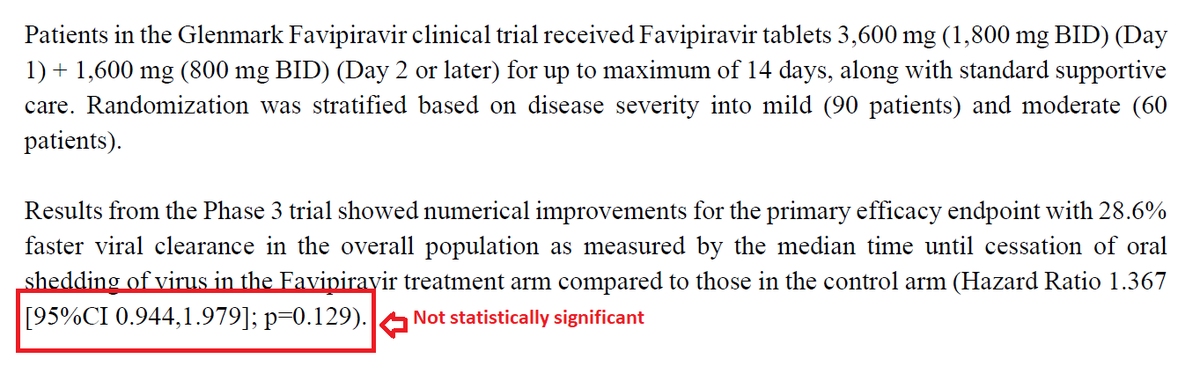
What did the researchers do?
In an ongoing multinational, placebo-controlled, observer-blinded trial, researchers randomly assigned 2260 adolescents to receive two injections, 21 days apart, of BNT162b2 vaccine or placebo.
What did the researchers do?
In an ongoing multinational, placebo-controlled, observer-blinded trial, researchers randomly assigned 2260 adolescents to receive two injections, 21 days apart, of BNT162b2 vaccine or placebo.

7/END
What are the key implications of the study?
Adolescents and community both benefit.
Schools are colleges can re-start.
What are the key implications of the study?
Adolescents and community both benefit.
Schools are colleges can re-start.

• • •
Missing some Tweet in this thread? You can try to
force a refresh