
Thread on #ProventionBio #Provention $PRVB and their flagship #antiCD3 monoclonal antibody #teplizumab under #FDA consideration for the “delay of clinical #Type1Diabetes #T1D in at-risk individuals”. I have no connection to the company – but I do have COIs. No recommendations
or claims of accuracy, just a summary of the status quo and personal opinion. Assume I’m biased. All public information. Feel free to correct me. For references & links, contact me. Some pictures are copies from public PRVB presentations/FDA briefing docs.
*Summary*
PRVB are seeking approval of 14-day IV teplizumab for the delay of T1D. Teplizumab is a humanised Fc-mutated anti-CD3 monocl Ab that, simply put, turns autoreactive T-cells (regardless of antigen) into exhausted T-cells
(PRVB corporate presentation slide)
PRVB are seeking approval of 14-day IV teplizumab for the delay of T1D. Teplizumab is a humanised Fc-mutated anti-CD3 monocl Ab that, simply put, turns autoreactive T-cells (regardless of antigen) into exhausted T-cells
(PRVB corporate presentation slide)

The FDA have granted BTD (Breakthrough Therapy) & Priority Review designation for PRVBs BLA. In May 2021, the AdComm (EMDAC – Endocrinol & Metabol Dx AdComm) voted 10 Yes vs. 7 No to recommend approval for the delay of clinical (Stage 3) T1D
based on a small phase 2 trial (TrialNet’s “TN-10” in 76 persons at risk of T1D) and supportive evidence from 5 previous trials in a different population (recent-onset T1D). Recall the staging model of (pre-)T1D by Insel et al. 2015:
Stage 1 = at least 2 islet antibodies, Stage 2 = plus dysglycaemia, Stage 3 = symptomatic hyperglycaemia i.e. clinical T1D.
(picture from PRVBs EMDAC slide deck:)
(picture from PRVBs EMDAC slide deck:)

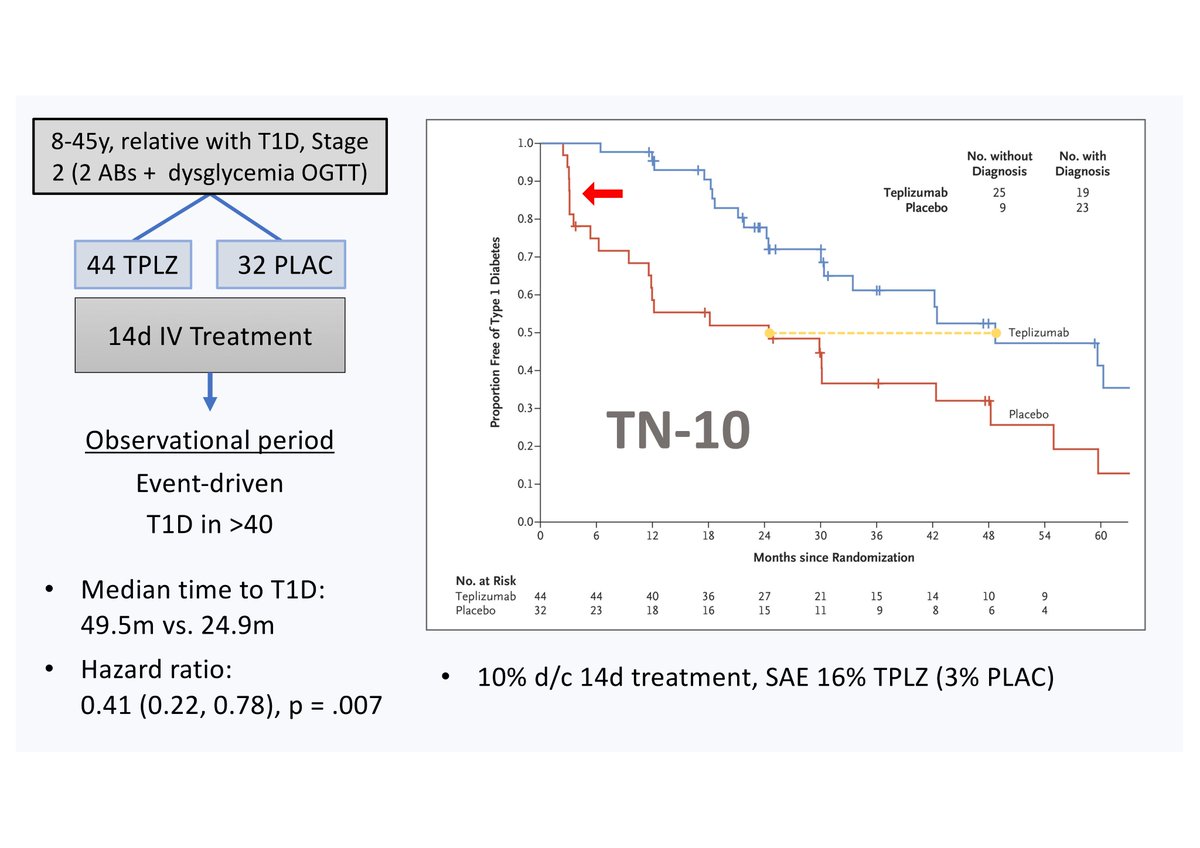
TN-10 was a phase II RCT of 8-45yo Stage 2 individuals with a family Hx of T1D (44 Teplz / 32 Placebo) that showed a median time to T1D of 49.5m (Teplz) vs. 24.9m (Plac), HR 0.41 (0.22, 0.78), p = .007.
(The recently published extension of TN-10 with longer follow-up was not part of the BLA so is not considered here. With the longer follow-up of median 2.5yrs it showed median times to T1D of 59.6m [Teplz] and 27.1m [Plac]).
Because of recruitment problems (TN-10 was active between 2011-2018!) the target N was halved and an event-driven end-of-study (40 cases of T1D) prespecified. As included in the BLA, TN-10 has a number of limitations such as:
(i) small N for a pivotal trial (only 44 Teplz-treated), (ii) short follow-up, (iii) no follow-up after T1D diagnosis, (iv) no ethnic diversity; (v) did not meet enrolment goals (target N halved); (vi) 2y endpt C-peptide not evaluable b/o different follow-ups between subjects.
Also, apart from the initial steep drop in the survival curve for the placebo group (red arrow), i.e., many placebo patients being diagnosed with T1D very soon after baseline, the curves are mostly parallel,
which might suggest that placebo subjects could have been “more” high-risk than Teplizumab-treated ones
(my slide)
(my slide)
In a single low-dose bridging study in healthy volunteers, PRVB failed to demonstrate PK/PD comparability between the TN-10 product (produced by Lilly/Macrogenics; PRVB had in-licensed Teplz in 2018 from Macrogenics) and the to-be-commercialized product.
(my slide)
(my slide)

PRVB argues that those differences (in AUC[0-inf]) are not clinically meaningful since the full Tx course (14-day IV Teplz) cannot be assessed wrt PK/PD comparability in a single low-dose study. They also say modelling suggests exposure above the clinical efficacy threshold.
The likely explanation for the lower AUC with the new product is faster clearance from the circulation. PRVB think it’s due to TMDD (target-mediated drug disposition). Teplizumab is a fast-acting AB (half-life 4 days). PRVB say the predominant mode of clearance
is by internalisation of the Teplizumab-bound CD3 receptor by T cells (i.e. TMDD). And that low and short doses such as in the bridging study result in fast clearance because they never reach the saturation point of CD3R’s, above which PRVB argue that the AUC differences
between the products disappear. They support this with (unpublished) PK data from the T1D Protégé trial that compared 14-day full dose, 14-day one third dose, and 6-day full dose. That trial (they say) also showed rapid clearance in the two short treatment arms.
Regardless,
On the PDUFA date, 2 July 2021, FDA sent a CRL faulting PRVB on the bridging study
On the PDUFA date, 2 July 2021, FDA sent a CRL faulting PRVB on the bridging study
and demanding demonstration of PK/PD comparability or data on why it doesn’t need to be demonstrated. FDA also noted “deficiencies conveyed during a recent general inspection” of the manufacturer. PRVB says the FDA did not comment on efficacy or safety of Teplz in the CRL.
PRVB’s plan is now to read out in Q3/2021 a PK/PD substudy of the ongoing PROTECT phase 3 trial (assessing 12-day IV teplizumab for C-peptide preservation in recent-onset T1D) to provide the FDA with PK/PD comparability data.
This would likely be a Class 2 re-submission of the BLA, with a max. 6 months turnaround time. So, if submission in Q4/2021, decision is expected in mid-2022.
Pertinent questions over the coming months are now:
Pertinent questions over the coming months are now:
(1) Will PRVB feel confident with the PROTECT PK/PD substudy results in Q3 and re-submit to the FDA? If so, will the new data satisfy the FDA regarding the comparability of the old and new drug products?
(2) Noted in the CRL were also that “deficiencies […] not specific to teplizumab, at a fill/finish manufacturing facility […] will need to be resolved before approval.” I’ve not seen further communication on this from PRVB – but this needs to be addressed of course.
(3) Approval or not: My feeling is the FDA might approve Teplizumab for the delay of T1D – or be it with requirements for post-marketing safety studies (?-marks e.g. long-term cancer risk, high DKA count in old ph. 3 trials) & maybe efficacy studies (replication of TN-10).
(to be continued in a second thread, seems like they don't allow more tweets in this one...)
I can like my own tweet? Sweeet. Problem solved.
This will make more sense:
Rachmaninov prélude op.23 no.4
This will make more sense:
Rachmaninov prélude op.23 no.4
• • •
Missing some Tweet in this thread? You can try to
force a refresh



