1/ On the heels of @ASCO #ASCO21 plenary, we are happy to share the results of KEYNOTE-564 trial of adjuvant pembrolizumab in mRCC just published in @NEJM ! A step towards better outcomes for all our patients with kidney cancer!
nejm.org/doi/full/10.10…
@OncoAlert @tompowles1
nejm.org/doi/full/10.10…
@OncoAlert @tompowles1

2/ Following nephrectomy for kidney cancer, a significant percentage of patients will experience disease recurrence, most of them with distant metastases, highlighting the need for effective adjuvant therapies.
3/ Despite many decades of research and clinical investigations, there are currently no globally approved adjuvant regimens for the management of #kidneycancer
4/ In an attempt to answer this unmet need, we conducted KEYNOTE-564, a phase 3 clinical trial of pembrolizumab vs. placebo, after nephrectomy and/or metastasectomy, in patients with ccRCC. Risk groups were defined as 1) pT2 grade4/sarc, T3, 2) pT4/N+, 3) M1NED within 1 year 

5/ The primary endpoint was disease-free survival (DFS). OS was a key secondary endpoint. Safety and patient-reported outcomes (PROs) were secondary/exploratory endpoints.
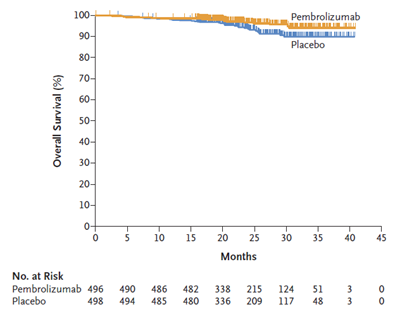
6/ A total of 994 patients were included, and were randomly assigned to receiving pembrolizumab (n=496) or placebo (n=498). The median duration of the trial regimen was 11.1 months in both groups.
7/ Median age was 60 years in both groups. In the two treatment arms, the majority of patients had M0 intermediate-high risk disease (>85%), followed by M0 high-risk (~7-8%) and M1 NED disease (5.8%). 

8/ Pembrolizumab significantly improved DFS as compared to placebo (HR=0.68 ; 95%CI: 0.53-0.87 ; p=0.002, 2-sided), with benefits identified across most key subgroups (all HR<1). DFS rates at 2 years were 77.3 and 68.1% in the pembrolizumab and placebo groups, respectively. 



9/ OS is very immature (HR=0.54; 95% CI: 0.30-0.96). Of note, only a small number of events (n=51) required for final analysis (n=200) had occurred. 
10/ Grade 3-4 AEs occurred in 32.4% of patients who received pembrolizumab versus 17.7% with placebo. Additionally, 20.7% of patients in the pembrolizumab arm discontinued treatment at any time due to AEs, compared to 2.0% in the placebo group. No deaths related to AEs occurred. 

11/ First very prelim look at QoL: no clinically meaningful changes in the FKSI-DRS and EORTC QLQ-C30 scores between groups.
12/ After more than TWENTY-NINE years of investigation (we can argue 1+ generation), KEYNOTE-564 represents the first positive trial of adjuvant immunotherapy in #kidneycancer.
We hope this work will help advance the field forward and offer better options for our patients.
We hope this work will help advance the field forward and offer better options for our patients.

13/ Many open questions:
-Which patients can be cured with surgery alone? ctDNA by conv. methods is challenging in RCC. I refer you to work with our work @DanaFarber, and recent work from @ZeynepZengin @montypal @neerajaiims
pubmed.ncbi.nlm.nih.gov/32341571/
pubmed.ncbi.nlm.nih.gov/34130999/
-Which patients can be cured with surgery alone? ctDNA by conv. methods is challenging in RCC. I refer you to work with our work @DanaFarber, and recent work from @ZeynepZengin @montypal @neerajaiims
pubmed.ncbi.nlm.nih.gov/32341571/
pubmed.ncbi.nlm.nih.gov/34130999/
14/-Who are the patients that do not respond to pembro and what to do? Intensify treatment by adding another “active drug” or try a new class of agents?
-How do sarcomatoid (and high-grade) patients do?
-How do sarcomatoid (and high-grade) patients do?
15/ -What are the Central Review results?
-Should we wait for OS, knowing DFS is acceptable per @US_FDA guidelines (watch @RanaMckay #ASCO21 stellar discussion @asco. Link: )
-Many more questions….and more follow-up will bring us closer to more answers.
-Should we wait for OS, knowing DFS is acceptable per @US_FDA guidelines (watch @RanaMckay #ASCO21 stellar discussion @asco. Link: )
-Many more questions….and more follow-up will bring us closer to more answers.
16/ I want to thank our collaborators, all the people involved in the achievement of this work including study staff and sponsor, and most importantly, our patients and their families who are our daily source of inspiration and for whom we dedicate all our efforts. #Onward!
• • •
Missing some Tweet in this thread? You can try to
force a refresh