To battle COVID, Biden's Action Plan boldy calls for scaling rapid tests! & requires millions of Americans to vaccinate or test
But now the timer is set. W/out immediate change, we will see PCR delays of 3-10 days & rapid test shelves remain largely empty
We can avoid this.
1/
But now the timer is set. W/out immediate change, we will see PCR delays of 3-10 days & rapid test shelves remain largely empty
We can avoid this.
1/
https://twitter.com/michaelmina_lab/status/1435367919865712644
Luckily, the issue is a simple one to fix (all things considered).
The tests exist in HUGE numbers across the globe. Just not in the US. The reason? We've asked the FDA to take on an impossible task... to evaluate high quality PUBLIC HEALTH tools, when this is not their job
2/
The tests exist in HUGE numbers across the globe. Just not in the US. The reason? We've asked the FDA to take on an impossible task... to evaluate high quality PUBLIC HEALTH tools, when this is not their job
2/
The FDA evaluates medical devices - and does not evaluate public health tools
This is THE problem. As long as we consider rapid tests as medical devices (they're not - they're PUBLIC HEALTH transmission detection tools) FDA is forced to fit a square peg in a round hole
3/
This is THE problem. As long as we consider rapid tests as medical devices (they're not - they're PUBLIC HEALTH transmission detection tools) FDA is forced to fit a square peg in a round hole
3/
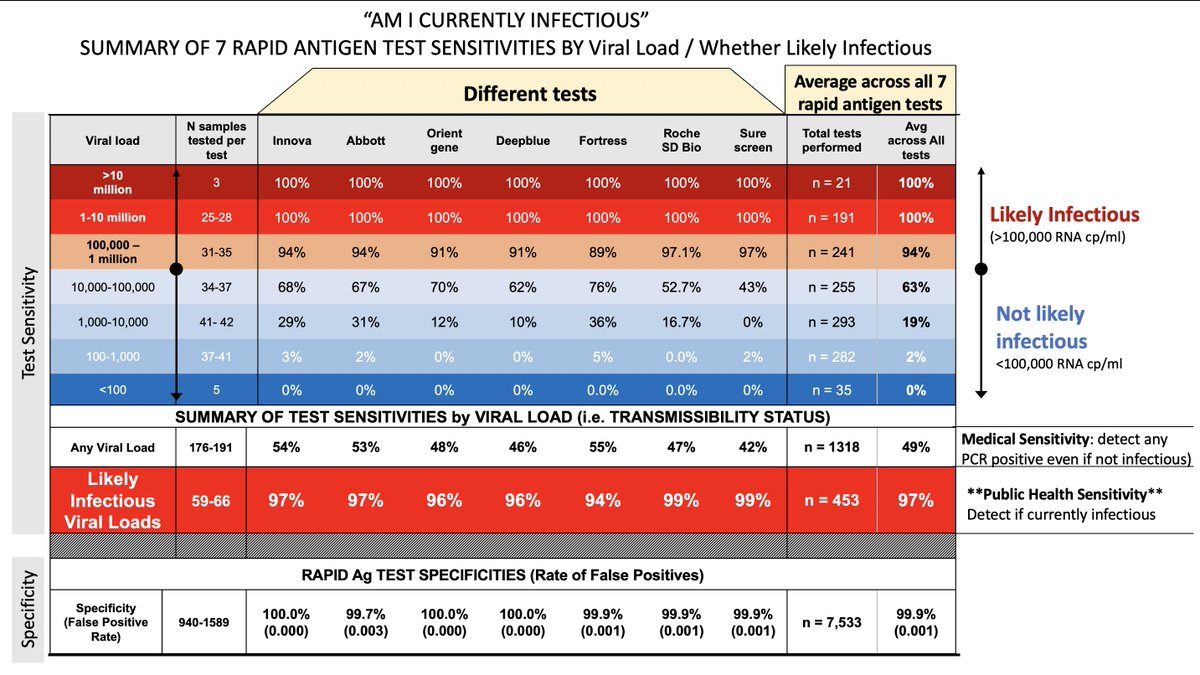
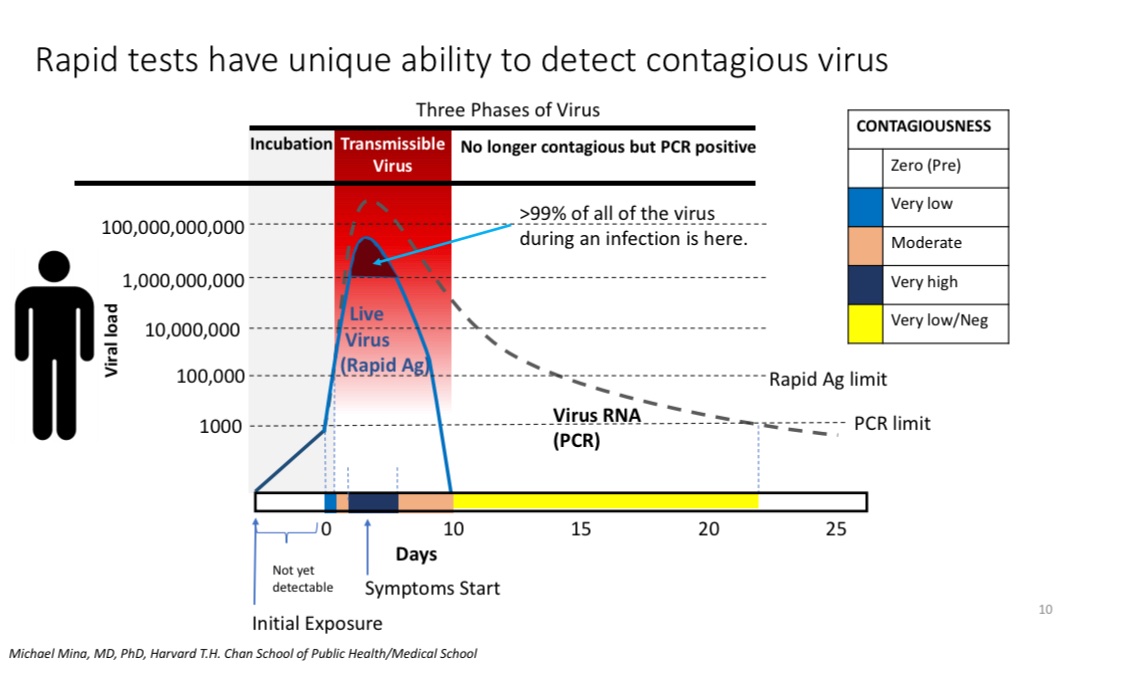
By evaluating as med devices, FDA is forced to ask tests (meant to be specific to contagiousness-a public health issue) to achieve sensitivity to tests that are specific simply to viral RNA, regardless of contagious levels or not. Thus, we've tasked the w an impossible task.
4/
4/
The simplest solution to increase access to tests in the US (and massively drop costs) is for the president to use executive actions that designate that
-tools used for public health testing, during the public health emergency, are public health tools, not medical devices.
5/
-tools used for public health testing, during the public health emergency, are public health tools, not medical devices.
5/
If this simple change is made, then FDA no longer tasked w evaluating rapid tests (since they don't evaluate public health tools) and can go to CDC/NIH to certify tests as appropriate for public health (i.e high sensitivity for viral loads that are a risk to transmit)
6/
6/
This allow allow certification of tests "by-right" based on their use in Europe, for instance, opening the US overnight to a massive influx of HIGH QUALITY rapid tests at low cost.
7/
7/
It will also allow very FAST EVALUTION of rapid tests using appropriate metrics - like sensitivity by viral load, using lab evaluations for preliminary certificatino, cutting time to market from 6-12 month clinical trials (that are not informative) to 2-5 day evaluations
8/
8/
• • •
Missing some Tweet in this thread? You can try to
force a refresh