
1/
Israel data: NEJM article
Booster group vs. 2-dose group.
BIG questions on results & methods.
“We considered 12 days as the interval between the administration of a booster dose and its likely effect on the observed number of confirmed infections”
nejm.org/doi/full/10.10…
Israel data: NEJM article
Booster group vs. 2-dose group.
BIG questions on results & methods.
“We considered 12 days as the interval between the administration of a booster dose and its likely effect on the observed number of confirmed infections”
nejm.org/doi/full/10.10…
2/
No. You can’t skip 12 days after booster, especially when there is evidence that the risk of infection is increased during the first 1-2 weeks post dose 1.
Reasons for increased early risk: immune suppression, infection at V sites.
No. You can’t skip 12 days after booster, especially when there is evidence that the risk of infection is increased during the first 1-2 weeks post dose 1.
Reasons for increased early risk: immune suppression, infection at V sites.
https://twitter.com/prof_shahar/status/1369823176197206019
3/
Happened in Israel, at both campaigns. Outbreaks in nursing homes.
Happened in Israel, at both campaigns. Outbreaks in nursing homes.
https://twitter.com/daridor/status/1424445833018236943
4/
Actually, they excluded more than half of the infections!
13K infected (Fig 1), only 5,373 analyzed (Table 2).

Actually, they excluded more than half of the infections!
13K infected (Fig 1), only 5,373 analyzed (Table 2).


5/
Moreover,
You can’t get to post 12-days benefit w/o passing through the effect during 1-12 days (possibly harmful).
(Just as you can’t get to 2nd dose w/o passing through 1st dose.)
Moreover,
You can’t get to post 12-days benefit w/o passing through the effect during 1-12 days (possibly harmful).
(Just as you can’t get to 2nd dose w/o passing through 1st dose.)
https://twitter.com/prof_shahar/status/1437851138712162304
6/
They report effects from regression models.
But crude (simple, unadjusted) estimates are similar.
Actually, we observe something called “negative confounding”. Adjusted > Unadjusted. Possible, but unusual.
They report effects from regression models.
But crude (simple, unadjusted) estimates are similar.
Actually, we observe something called “negative confounding”. Adjusted > Unadjusted. Possible, but unusual.

7/
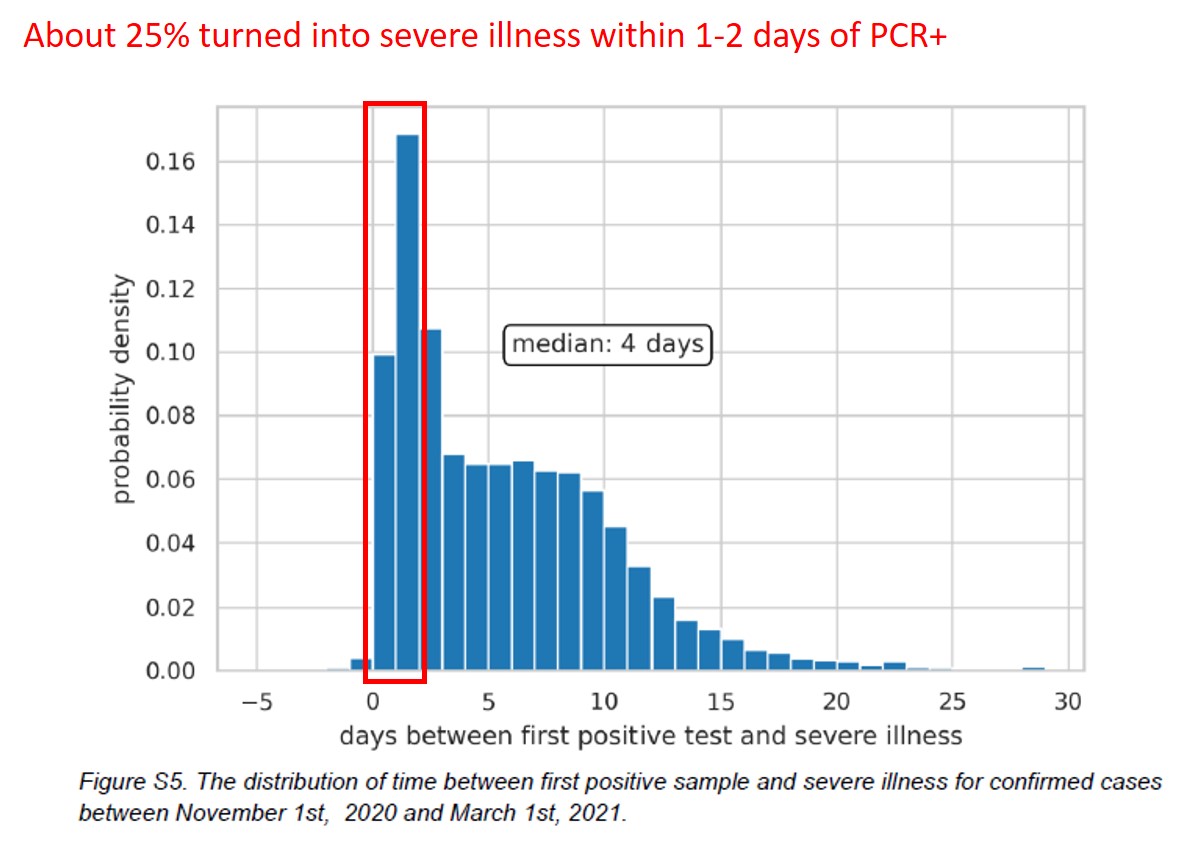
Next, effect on severe cases.
Figure refers to winter wave? (typo?)
About 25% became severe within 1-2 days of PCR+. Strange.
Likely, first PCR+ after admission (for another reason?)
That anomaly observed before. Source of detection bias?

Next, effect on severe cases.
Figure refers to winter wave? (typo?)
About 25% became severe within 1-2 days of PCR+. Strange.
Likely, first PCR+ after admission (for another reason?)
That anomaly observed before. Source of detection bias?
https://twitter.com/prof_shahar/status/1371634315679862787
8/
Estimated effects by days since booster.
Do you see a downward trend that was visually saved by day 25 (with a huge CI)?
Is this a true pattern?
Should we not gather more data to see if the downward trend continues and where it ends?
Average over 12-25 days is not good enough
Estimated effects by days since booster.
Do you see a downward trend that was visually saved by day 25 (with a huge CI)?
Is this a true pattern?
Should we not gather more data to see if the downward trend continues and where it ends?
Average over 12-25 days is not good enough

10/
To sum up:
Considering these questions, does FDA have enough unequivocal evidence to certify 3rd dose?
In the pre-COVID era: No
But this is Newscience.
>>More (bonus 1-3)
To sum up:
Considering these questions, does FDA have enough unequivocal evidence to certify 3rd dose?
In the pre-COVID era: No
But this is Newscience.
>>More (bonus 1-3)
• • •
Missing some Tweet in this thread? You can try to
force a refresh









