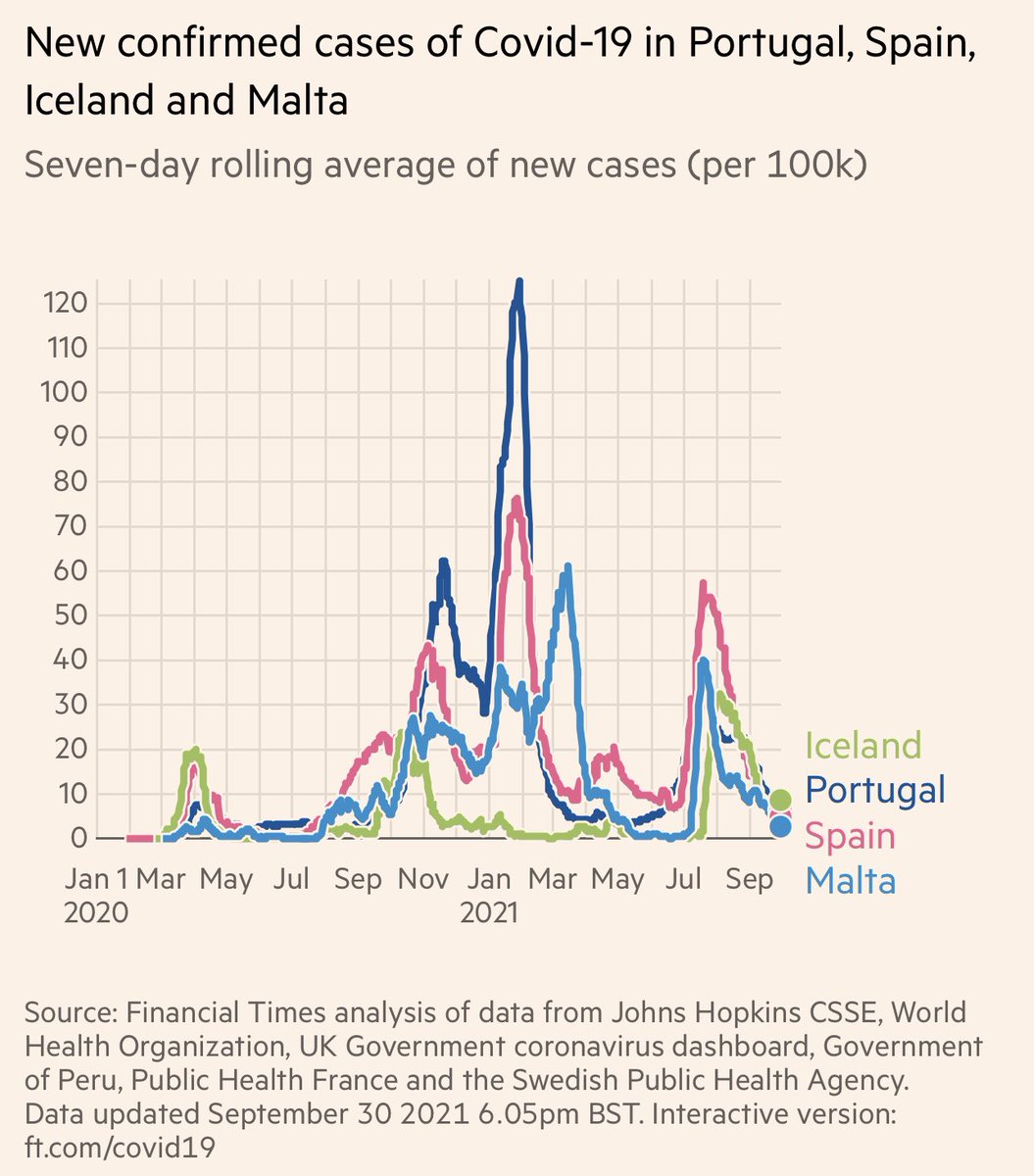
Medicare Part D spends 60% of its money on 250 drugs that have only one manufacturer and no generic or biosimilar competitors. #UnregulatedMonopoly
13% on 2,208 other drugs with only one manufacturer
27% on 1,078 drugs with more than one manufacturer.
kff.org/medicare/issue…
13% on 2,208 other drugs with only one manufacturer
27% on 1,078 drugs with more than one manufacturer.
kff.org/medicare/issue…

Not all drugs with only one manufacturer are a monopoly. That's why some of single source drugs are lower priced. If an alternative drug is available from another manufacturer, there's competition; many equivalent drugs, especially generic equivalents, means real competition.
On the other hand, if a new cancer drug works for only a few months or years, and then stops working - the case with most cancer medicines - then even if there are multiple meds EACH drug remains a monopoly. Because we want to try each drug and prolong life the most we can.
Monopoly is a huge problem with cancer drugs when a new drug is needed for survival and only one manufacturer makes that medicine. Since Medicare is prohibited from negotiating, the manufacture dictates whatever price they can get away with. Public outrage is the only barrier.
You can also have oligopoly. 3 companies control most of the insulin market in the US. So insulin prices rise lock step. When one company increases the price, the others do as well. It's effective monopoly. No collusion needed. Just tacit understanding.
• • •
Missing some Tweet in this thread? You can try to
force a refresh