👍🙂💊SARSCoV2 protease inhibitor Paxlovid approved as the first pill for preventing severe COVID19 i the US.
This is great news, as it prevents hospitalizations by 89% in high-risk patients with SARSCoV2 infections.
This is great news, as it prevents hospitalizations by 89% in high-risk patients with SARSCoV2 infections.
https://twitter.com/CNN/status/1473710517697056778
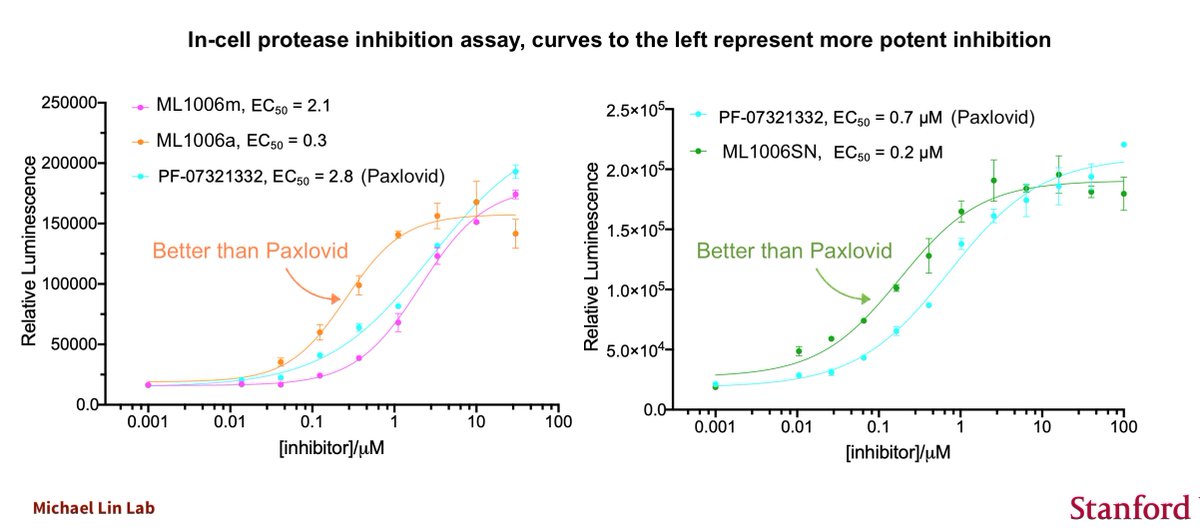
And I'm obligated to point out that we were the first to invent a SARSCoV2 protease inhibitor with the essential elements later found in Paxlovid (we showed our drugs in 9/2020, Pfizer announced Paxlovid in 4/2021)
https://twitter.com/michaelzlin/status/1306271055313424390
It's commonly misreported that Pfizer was able to make Paxlovid quickly because they had SARSCoV1 protease inhibitors from 2005. That is absolutely false. Paxlovid did not derive from their prior work. It was derived from our or other labs' work in 2020.
https://twitter.com/michaelzlin/status/1462963595193602052
Pfizer's earlier SARSCoV2 protease inhibitor, PF-00835231, indeed came from their earlier SARSCoV1 work. But this is an IV-only drug and testing was stopped after Pfizer saw our work (or others') that the HCV protease inhibitor boceprevir can be modified into a SARSCoV2 inhibitor
That's why Pfizer's drug is chemically similar to our ML1000 announced in September 2020. Most of our drug, and theirs, derives from the off-patent boceprevir., which we and other academic labs found to have the right shape to bind the SARSCoV2 protease.
https://twitter.com/michaelzlin/status/1462963595193602052
So I'm happy that Pfizer made Paxlovid, as it's a good drug. I'm also happy that academic labs such as ours provided information that pharma companies could use to accelerate the search for treatments. It's what academics do.
Thus it's important to recognize the role of academic research in Paxlovid's development. We've already acknowledged it for vaccine development, so really it should be no surprise it can also occur for therapeutics.
And many academics are doing COVID19 work on a volunteer basis. My lab has yet to receive any funding from NIH for our protease inhibitor research, for example. And we explore questions big pharma thinks aren't productive, and we make our findings public. That's an excellent ROI.
We received funding for SARSCoV2 work from Fast Grants from @patrickc, @Stanford_ChEMH, and the @Stanford Coulter program. It's been enough funding for 1 person essentially, vs 300 scientists Pfizer has put on it.
In short, where did Pfizer's ideas come from? See and decide for yourself.
History A: as told by Pfizer
History B: more parsimonious (typical academic understatement)
History A: as told by Pfizer
History B: more parsimonious (typical academic understatement)

• • •
Missing some Tweet in this thread? You can try to
force a refresh