
I've been meaning to reveal some exciting results from my lab's SARSCoV2 protease inhibitor research. With FDA approval of Pfizer's Paxlovid today, it's a good time to finally share our good news as well.
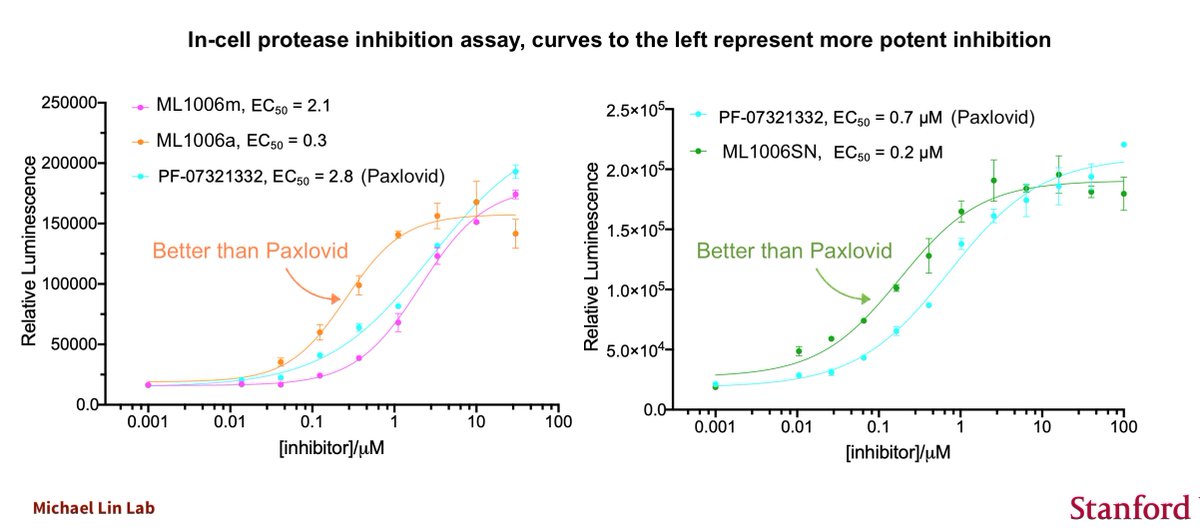
We've developed not 1 but 2 inhibitors with activity superior to Paxlovid.
We've developed not 1 but 2 inhibitors with activity superior to Paxlovid.
This work began in March 2020 when we hypothesized the HCV protease inhibitor boceprevir could serve as a template for the creation of orally dosable SARSCoV2 protease inhibitors. We announced initial results from this work in September 2020.
https://twitter.com/michaelzlin/status/1306271055313424390
Our initial drug, ML1000, described in 2020 September, preceded Pfizer's PF-07321332 (Paxlovid) announced in 2021 April and approved today. Other groups have also made drugs with boceprevir parts, but our drug is the closest in structure to PF-07321332 

Our two best drugs, ML1006a and ML1006SN, derive from ML1000 as well, and are thus siblings of Paxlovid. We are working on even further improvements while we discuss commercialization possibilities.
I'll come back with more info in the future but the thread below explains our drug development process. It is a story of being prepared for serendipity
https://twitter.com/michaelzlin/status/1456734281011634177
• • •
Missing some Tweet in this thread? You can try to
force a refresh






